For further reading see:
 "Synthesis, characterization and stability of phosphonium phenolate zwitterions derived from a (diphenylphosphino)phenol derivative and oxiranes."
"Synthesis, characterization and stability of phosphonium phenolate zwitterions derived from a (diphenylphosphino)phenol derivative and oxiranes."
Steiner, M. R.; Marschner, C.; Baumgartner, J.; Hlina, J. A.; Slugovc, C. Monatsh. Chem. 2024, 155, 715-723. DOI: 10.1007/s00706-024-03216-1
 "Using the phospha-Michael reaction for making phosphonium phenolate zwitterions."
"Using the phospha-Michael reaction for making phosphonium phenolate zwitterions."
Steiner, M. R.; Schmallegger, M.; Donner, L.; Hlina, J. A.; Marschner, C.; Baumgartner, J.; Slugovc, C. Beilstein J. Org. Chem. 2024, 20, 41–51. DOI: 10.3762/bjoc.20.6
 "Functionalized electron-rich pyridines as initiators for the epoxy homopolymerization."
"Functionalized electron-rich pyridines as initiators for the epoxy homopolymerization."
Edinger D.; Fischer, S. M.; Slugovc, C. Macromol. Chem. Phys. 2024, 225, 2300299. DOI: 10.1002/macp.202300299
 "Poly(ether)s derived from oxa-Michael polymerization - A comprehensive review."
"Poly(ether)s derived from oxa-Michael polymerization - A comprehensive review."
Ratzenböck, K.; Fischer, S. M.; Slugovc, C. Monatsh. Chem. 2023, 154, 443-458. DOI: 10.1007/s00706-023-03049-4
 "Sequential dual-curing of electron-deficient olefins and alcohols relying on oxa-Michael addition and anionic polymerization"
"Sequential dual-curing of electron-deficient olefins and alcohols relying on oxa-Michael addition and anionic polymerization"
Fischer, S. M.; Schallert, V.; Uher, J. M.; Slugovc, C. Polym. Chem. 2023, 14, 1081-1084. DOI: 10.1039/D3PY00035D
 "Exploiting retro oxa-Michael chemistry in polymers."
"Exploiting retro oxa-Michael chemistry in polymers."
Ratzenböck, K.; Uher, J. M.; Fischer, S. M.; Edinger, D.; Schallert, V.; Žagar, E.; Pahovnik, D.; Slugovc, C. Polym. Chem. 2023, 14, 651-661. DOI: 10.1039/D2PY01345B
 "Tris(2,4,6-trimethoxyphenyl)phosphine - a Lewis base able to compete with phosphazene bases in catalysing oxa-Michael reactions."
"Tris(2,4,6-trimethoxyphenyl)phosphine - a Lewis base able to compete with phosphazene bases in catalysing oxa-Michael reactions."
Fischer, S. M.; Kaschnitz, P.; Slugovc, C. Catal. Sci. Technol. 2022, 12, 6204-6212. DOI: 10.1039/D2CY01335E
 "Water as a monomer: synthesis of an aliphatic polyethersulfone from divinyl sulfone and water."
"Water as a monomer: synthesis of an aliphatic polyethersulfone from divinyl sulfone and water."
Ratzenböck, K.; Din, M. M. U.; Fischer, S. M.; Žagar, E.; Pahovnik, D.; Boese, A. D.; Rettenwander, D.; Slugovc, C. Chem. Sci. 2022, 13, 6920-6928. DOI: 10.1039/D2SC02124B
 "Electron-rich triarylphosphines as nucleophilic catalysts for oxa-Michael reactions."
"Electron-rich triarylphosphines as nucleophilic catalysts for oxa-Michael reactions."
Fischer, S. M.; Renner, S.; Boese, A. D.; Slugovc, C. Beilstein J. Org. Chem. 2021, 17, 1689–1697. DOI: 10.3762/bjoc.17.117
 "Melt-polymerization of acrylamide initiated by nucleophiles: a route towards highly branched and amorphous polyamide 3."
"Melt-polymerization of acrylamide initiated by nucleophiles: a route towards highly branched and amorphous polyamide 3."
Edinger, D.; Weber, H.; Žagar, E.; Pahovnik, D.; Slugovc, C. ACS Appl. Polym. Mater. 2021, 3, 2018-2026. DOI: 10.1021/acsapm.1c00084
 "Step-growth polymerisation of alkyl acrylates via concomitant oxa-Michael and transesterification reactions."
"Step-growth polymerisation of alkyl acrylates via concomitant oxa-Michael and transesterification reactions."
Ratzenböck, K.; Pahovnik, D.; Slugovc, C. Polym. Chem. 2020, 11, 7476-7480. DOI: 10.1039/D0PY01271H
 "Solvent‐ and catalyst‐free aza‐Michael addition of imidazoles and related heterocycles."
"Solvent‐ and catalyst‐free aza‐Michael addition of imidazoles and related heterocycles."
Kodolitsch, K.; Gobec, F.; Slugovc, C. Eur. J. Org. Chem. 2020, 19, 2973-2978. DOI: 10.1002/ejoc.202000309
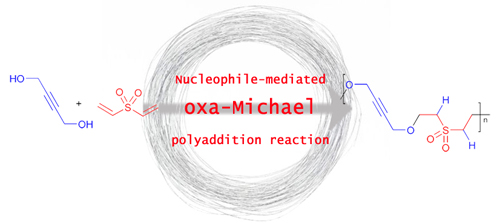
 "Solvent-free macrocyclisation by nucleophile-mediated oxa-Michael addition polymerisation of divinyl sulfone and alcohols."
"Solvent-free macrocyclisation by nucleophile-mediated oxa-Michael addition polymerisation of divinyl sulfone and alcohols."
Strasser, S.; Wappl. C.; Slugovc. C. Polym. Chem. 2017, 8, 1797-1804. DOI: 10.1039/C7PY00152E - see the feature for more information.
 "Nucleophile-mediated oxa-Michael addition reactions of divinyl sulfone - a thiol-free option for step-growth polymerisations."
"Nucleophile-mediated oxa-Michael addition reactions of divinyl sulfone - a thiol-free option for step-growth polymerisations."
Strasser, S.; Slugovc, C. Catal. Sci. Technol. 2015, 5, 5091-5094. DOI: 10.1039/c5cy01527h
 "Synthesis, characterization and stability of phosphonium phenolate zwitterions derived from a (diphenylphosphino)phenol derivative and oxiranes."
"Synthesis, characterization and stability of phosphonium phenolate zwitterions derived from a (diphenylphosphino)phenol derivative and oxiranes." "Using the phospha-Michael reaction for making phosphonium phenolate zwitterions."
"Using the phospha-Michael reaction for making phosphonium phenolate zwitterions." "
" "Sequential dual-curing of electron-deficient olefins and alcohols relying on oxa-Michael addition and anionic polymerization"
"Sequential dual-curing of electron-deficient olefins and alcohols relying on oxa-Michael addition and anionic polymerization" "Exploiting retro oxa-Michael chemistry in polymers."
"Exploiting retro oxa-Michael chemistry in polymers." "Tris(2,4,6-trimethoxyphenyl)phosphine - a Lewis base able to compete with phosphazene bases in catalysing oxa-Michael reactions."
"Tris(2,4,6-trimethoxyphenyl)phosphine - a Lewis base able to compete with phosphazene bases in catalysing oxa-Michael reactions." "Water as a monomer: synthesis of an aliphatic polyethersulfone from divinyl sulfone and water."
"Water as a monomer: synthesis of an aliphatic polyethersulfone from divinyl sulfone and water." "Melt-polymerization of acrylamide initiated by nucleophiles: a route towards highly branched and amorphous polyamide 3."
"Melt-polymerization of acrylamide initiated by nucleophiles: a route towards highly branched and amorphous polyamide 3." "Step-growth polymerisation of alkyl acrylates via concomitant oxa-Michael and transesterification reactions."
"Step-growth polymerisation of alkyl acrylates via concomitant oxa-Michael and transesterification reactions." "Solvent‐ and catalyst‐free aza‐Michael addition of imidazoles and related heterocycles."
"Solvent‐ and catalyst‐free aza‐Michael addition of imidazoles and related heterocycles." "
" "Nucleophile-mediated oxa-Michael addition reactions of divinyl sulfone - a thiol-free option for step-growth polymerisations."
"Nucleophile-mediated oxa-Michael addition reactions of divinyl sulfone - a thiol-free option for step-growth polymerisations."