Methods
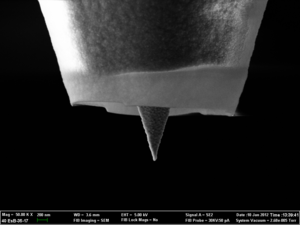
Technical surfaces usually are polycrystalline and often vary locally in structure and composition. This can result in a strong variation of local reactivity, e.g. in galvanic corrosion. But even electrode surfaces with highly homogeneous composition often show strongly varying local reaction rates in electrochemical processes as a result of a non-uniform potential distribution. The local reactivity of such surfaces can be mapped by scanning electrochemical microscopy (SECM), which uses an ultramicroelectrode that amperometrically detects a redox-active molecule reacting at the surface, while it rasters across it. The current signal not only depends on the local reactivity but also on the distance of the tip of the ultramicroelectrode from that surface. It is thus necessary to decouple the variation of the current caused by a change of the local tip-substrate distance (due to the substrate topography) from that caused by the change in local reactivity of the sample. We have joined forces with colleagues from Solid-State Electronics at the Vienna University of Technology and the University of Bern to develop Atomic Force Microscopy (AFM) tips with integrated nanoelectrodes. Such bifunctional tips can be used in conventional AFM instruments to map topography and simultaneously record reactivity maps of the sample surface immersed in an electrolyte. The amperometric response of these tips has been modelled by our collaborators at the University of Southampton.
I. Pobelov, M. Mohos, K. Yoshida, V. Kolivoska, A. Avdic, A. Lugstein, E. Bertagnolli, K. Leonhardt, G. Denuault, B. Gollas, T. Wandlowski, "Electrochemical current-sensing atomic force microscopy in conductive solutions", Nanotechnology, 2013, 24, 115501-115510. DOI: 10.1088/0957-4484/24/11/115501
K. Leonhardt, A. Avdic, A. Lugstein, I. Pobelov, T. Wandlowski, B. Gollas, G. Denuault, "Scanning electrochemical microscopy: Diffusion controlled approach curves for conical AFM-SECM tips", Electrochem. Commun., 2013, 27, 29-33. DOI: 10.1016/j.elecom.2012.10.034
K. Leonhardt, A. Avdic, A. Lugstein, I. Pobelov, T. Wandlowski, M. Wu, B. Gollas, G. Denuault, "Atomic Force Microscopy - Scanning Electrochemical Microscopy: Influence of tip geometry and insulation defects on diffusion controlled currents at conical electrodes", Anal. Chem., 2011, 83, 2971-2977. DOI: 10.1021/ac103083y
A. Avdic, A. Lugstein, M. Wu, B. Gollas, I. Pobelov, T. Wandlowski, K. Leonhardt, G. Denuault, E. Bertagnolli, "Fabrication of cone-shaped boron doped diamond and gold nanoelectrodes for AFM-SECM", Nanotechnology, 2011, 22, 145306. DOI: 10.1088/0957-4484/22/14/145306
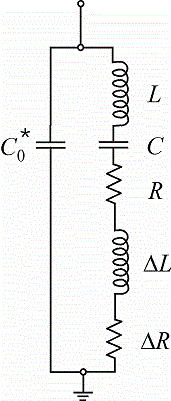
Electrode reactions often result in a change of the mass of the electrode, e.g. during electrodeposition or -dissolution. Such tiny mass changes (in the range of micro- to nanograms) can be monitored as a frequency change, if the electrode is coated on an oscillating shear-thickness resonator (e.g. a piezoelectric quartz-crystal). Such a setup is known as electrochemical quartz-crystal microbalance (EQCM). In many cases mass changes are accompanied by changes in the viscoelastic properties of either the electrode, e.g. in the case of polymer films, or of the electrolyte. The frequency of the oscillating electrode also varies with viscoelastic changes. Changes of mass and viscoelastic properties can be distinguished, if the electroacoustic impedance of the electrode is monitored instead of the frequency, which constitutes only the imaginary part of that complex impedance. We thus use homemade EQCM equipment that measures the electroacoustic impedance and analyze the data in terms of the damping resistance and the reactive inductance in a modified Butterworth-Van-Dyke equivalent circuit. Such an EQCM can additionally be combined with an SECM. The ultramicroelectrode can then probe the composition of the diffusion layer next to the substrate electrode and e.g. identify targeted redox-active species.
B. Gollas, P. N. Bartlett, G. Denuault, “An instrument for simultaneous EQCM impedance and SECM measurements”, Anal. Chem., 2000, 72, 349-356. DOI: 10.1021/ac990796o
B. Gollas, J. M. Elliott, P. N. Bartlett, “Electrodeposition and properties of nanostructured platinum films studied by quartz crystal impedance measurements at 10 MHz”, Electrochim. Acta, 2000, 45, 3711-3724. DOI: 10.1016/S0013-4686(00)00464-3
Institute for Chemistry and Technology of Materials
Stremayrgasse 9
A-8010 Graz
Phone: +43 (0)316 873-32338
bernhard.gollas@tugraz.at