Setting the stage for the oxa-Michael addition polymerization
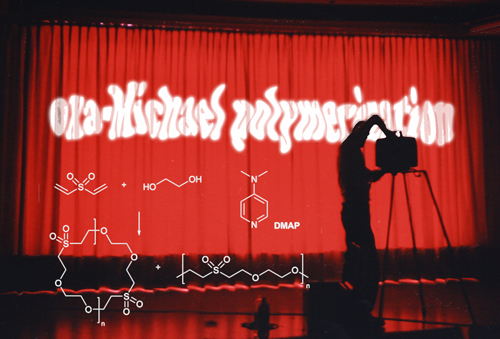
Researchers of ICTM circumstantiate in their latest paper the potential of the oxa-Michael-addition reaction as a tool in polymer synthesis. Following a preliminary communication (Catal. Sci. Technol. 2015, 5, 5091-5094), the current work introduces 4-dimethylaminopyridine (DMAP) as the initiator, which induces the addition polymerization of
alcohols (as the Michael-donor) and divinyl sulfone (as the Michael acceptor). This atom economic polymerization reaction proceeds quickly and quantitatively under solvent-free conditions. Upon prolonged curing predominantly macrocyclic products are formed from difunctional alcohols. This finding is highly counter-intuitive since macrocyclization reactions are usually performed in diluted solutions. Using trifunctional alcohols such as glycerol lead to duroplastic polymers. With these results authors demonstrated for the first time that the oxa-Michael addition polymerization qualifies as an attractive alternative to the well-established thiol-Michael variant.
For further reading see the open-access article:

"
Solvent-free macrocyclisation by nucleophile-mediated oxa-Michael addition polymerisation of divinyl sulfone and alcohols."
Strasser, S.; Wappl. C.; Slugovc. C.
Polym. Chem. 2017,
8, 1797-1804.
DOI: 10.1039/C7PY00152E