Reaction mechanisms
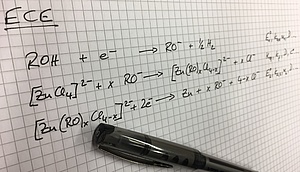
Electrochemistry covers all types of charge transfer reactions at interfaces. Electrochemical reactions are ubiquitous in daily life and play an important role in corrosion, batteries, fuel cells, photovoltaics, sensors, surface finishing, water purification, the recycling of chemicals and materials, and in the production of commodities. For efficient applications of electrochemical reactions a fundamental understanding of their thermodynamics and kinetics is essential.
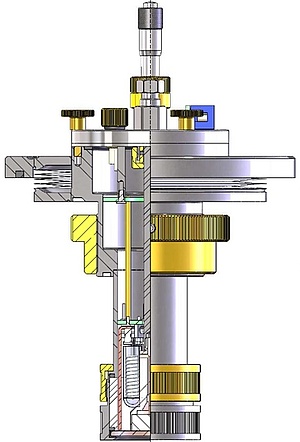
Apart from heterogeneous charge transfer, elementary steps in electrode reactions may include mass transport, adsorption, phase formation and coupled chemical reactions. The Gollas group uses a range of classical electrochemical methods to study electrode reactions in aqueous as well as non-aqueous electrolytes. While electrochemical methods like cyclic voltammetry or chronoamperometry are well suited to study thermodynamic and kinetic parameters of electrochemical reactions, they provide no information on the chemical identity of the reacting species. Therefore, we combine electrochemical with structure-sensitive methods such as UV/Vis, IR, Raman, NMR spectroscopy and small angle X-ray scattering. If necessary and possible, these structure-sensitive methods are applied in-situ, i.e. in the electrochemical cell, or directly coupled.
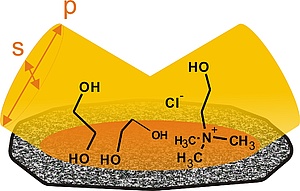
Recently, our focus of mechanistic studies has been on the electrodeposition of metals and alloys from deep eutectic solvents. Deep eutectic solvents are mixtures of inorganic or organic salts with hydrogen bond donors and share many of the useful properties of room temperature ionic liquids. In addition, they are considered as truly "green", because their constituents are environmentally benign. This is the case e.g. for mixtures of choline chloride with ethylene glycol. We have used this electrolyte for the electrodeposition of zinc. Due to its negative standard potential, aqueous electroplating of zinc is usually accompanied by massive evolution of hydrogen. This causes hydrogen embrittlement in many substrates, like higher strength steels and necessitates costly post-treatments. In contrast to aqueous electrolytes, zinc can be electroplated from deep eutectic solvents without the concomitant evolution of hydrogen, if the zinc speciation is controlled by the addition of alkoxy ions. We are the first, who combined electrochemistry with PM-IRRAS on glassy carbon electrodes to understand the complex reaction mechanism of zinc electrodeposition in this deep eutectic solvent.
P.A Wieser, D. Moser, B. Gollas, H. Amenitsch, “Monitoring of Pore Orientation by In Operando Grazing Incidence Small Angle X-ray Scattering during Templated Electrodeposition of Mesoporous Pt films”, ACS Appl. Mater. Interfaces, 2023, 15, 47604–47614. DOI: 10.1021/acsami.3c03316
D. Fuchs, B.C. Bayer, T. Gupta, G.L. Szabo, R.A. Wilhelm, D. Eder, J.C. Meyer, S. Steiner, B. Gollas, "Electrochemical behavior of graphene in a deep eutectic solvent", ACS Appl. Mater. Interfaces, 2020, 12, 40937-40948. DOI: 0.1002/open.201700045
L. Vieira, J. Burt, P.W. Richardson, D. Schloffer, D. Fuchs, A. Moser, P.N. Bartlett, G. Reid, B. Gollas, "Tin, bismuth, and tin-bismuth alloy electrodeposition from chlorometalate salts in deep eutectic solvents", ChemistryOpen, 2017, 6, 393-401. DOI: 10.1002/open.201700045
L. Vieira, R. Schennach, B. Gollas, "The effect of the electrode material on zinc electrodeposition from deep eutectic solvents", Electrochim. Acta, 2016, 197, 344-352. DOI: 10.1016/j.electacta.2015.11.030
L. Vieira, R. Schennach, B. Gollas, "In situ PM-IRRAS of a glassy carbon electrode/deep eutectic solvent interface", Phys. Chem. Chem. Phys., 2015, 17, 12870-12880. DOI: 10.1039/c5cp00070j
L. Vieira, A. H. Whitehead, B. Gollas, "Mechanistic studies of zinc electrodeposition from deep eutectic electrolytes", J. Electrochem. Soc., 2014, 161, D7-D13. DOI: 10.1149/2.016401jes
A. H. Whitehead, M. Pölzler, B. Gollas, "Zinc electrodeposition from a deep eutectic system containing choline chloride and ethylene glycol", J. Electrochem. Soc., 2010, 157, D328-D334. DOI: 10.1149/1.3364930
Institute for Chemistry and Technology of Materials
Stremayrgasse 9
A-8010 Graz
Phone: +43 (0)316 873-32338
bernhard.gollas@tugraz.at