Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors
Typically some seven orders of magnitude lower ion diffusivity in solids vs. in liquids is a key reason for vastly differing kinetics of their electrochemical reactions. Yet the solid state is considered to be required for high density of redox groups. Solubility limitations restrict their density in liquid electrolytes to typically 1/50 to 1/1000 of that in solids. With regard to energy storage devices this leads to high-energy batteries and high-power supercapacitors with the well-known large deficiency in the other property. Fast reactions at the surface rather than slow bulk solid reactions are deemed to be required for the high power of supercapacitors. Attempts to improve their energy involve pseudocapacitive Faradaic storage at the porous electrode material surface in addition to capacitive, equally surface governed double layer storage.
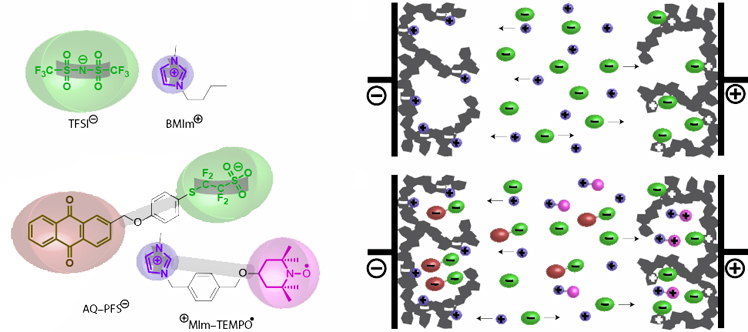
Here a group of researchers from the universities of Montpellier, Bordeaux and the ICTM show that towards bulk like redox density at liquid like fast kinetics can be achieved with biredox ionic liquids that we introduce in this work. These are functional ionic liquids (IL) where both cation and anion bear moieties that can undergo very fast reversible redox reactions. We show their properties in supercapacitors, which represent a first demonstration of the unique possibilities of biredox ionic liquids that can more generally enable high-capacity / high-rate charge storage. Such biredox ionic liquids enable combining an array of properties: (i) accessing towards bulk like redox density at liquid like fast kinetics, (ii) surmounting the solubility limit of conventional redox species towards bulk redox density in the liquid state, and when applied to capacitors (iii) decoupling electrostatic and pseudocapacitive charge storage from the ion accessible surface area, (iv) retaining the redox species in the electrodes to minimize self-discharge and leak current, and (v) raising working voltage due to the wide electrochemical window of IL electrolyte.
For more information see the according
press-release and the original article:

"Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors."
Mourad, E.; Coustan, L.; Lannelongue, P.; Zigah, D.; Mehdi, A.; Vioux, A.; Freunberger, S. A.; Favier, F.; Fontaine, O.
Nat. Mater. 2017,
in press.
DOI: 10.1038/nmat4808