New communication on click chemistry in OBC
Click chemistry has definitely found its place in the macromolecular chemistry toolbox and several reactions have been successfully applied for the synthesis of novel macromolecules and hybrid materials. To further advance this exciting field, the combination of multiple “click” reactions has emerged as an urgent issue to be addressed. Recent approaches involve the use of different catalysts, protecting groups or purification steps between the different click reactions to allow multiple conjugations. It is, however, of utmost importance to develop reaction protocols, which do not require such additional steps, for the synthesis of hybrid materials, especially when surfaces are modified or polymers are employed and byproducts cannot be easily removed.
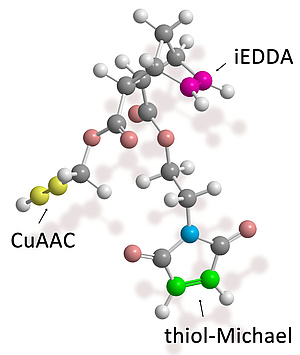
Therefore, to accommodate three different, well-established click reactions, we prepared a small trifunctional linker molecule with three different functional groups subjectable to Michael thiol-ene, azide-alkyne and tetrazine-alkene click chemistry. We hypothesized that these three reactions should interfere only minimally with each other, so that a “triple-click” product would be feasible. The individual click reactions proceeded with good conversion and with good selectivity as underlined by MALDI-TOF-MS and 1H-NMR spectroscopy. By simply performing these three reactions in the correct order in one pot, the target “triple clicked” product could be obtained in good yield and purity without requiring any additional purification or modification steps which is a significant advance to currently available approaches.
For more information see the open-access article: "A trifunctional linker suitable for conducting three orthogonal click chemistries in one pot."
"A trifunctional linker suitable for conducting three orthogonal click chemistries in one pot."
Knall, A.-C.; Hollauf, M.; Saf, R.; Slugovc, C. Org. Biomol. Chem. 2016, 14, 10576-10580. DOI: 10.1039/C6OB02182D
For more information see the open-access article:
 "A trifunctional linker suitable for conducting three orthogonal click chemistries in one pot."
"A trifunctional linker suitable for conducting three orthogonal click chemistries in one pot." Knall, A.-C.; Hollauf, M.; Saf, R.; Slugovc, C. Org. Biomol. Chem. 2016, 14, 10576-10580. DOI: 10.1039/C6OB02182D