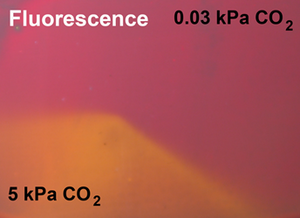
Figure 4.1. Photographic image of the carbon dioxide sensor based on diketo-pyrrolo-pyrrole dye embedded into ethylcellulose under UV excitation. Reprodcued from [25].
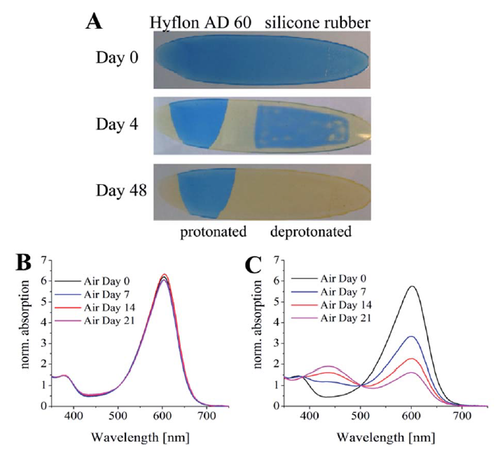
Figure 4.2. Protective properties of silicone rubber and Hyflon AD 60. (A) Photographic images of the planar optode based on m-cresol purple and TOAOH in ethyl cellulose covered with the protective layers; (B) and (C) corresponding UV-VIS absorption spectra for the Hyflon AD 60 and silicone rubber, respectively. Reprodcued from [28].
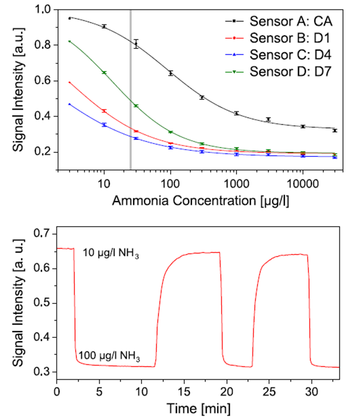
Figure 4.3. Up: calibration plots for ammonia sensors ammonia sensors based on cellulose acetate (CA) and different polyurethane hydrogels with the indication of the ammonia toxicity level in fish of 25 μg/l (grey line); below: response and recovery of sensor B between 10 and 100 μg/l ammonia in 100 mM phosphate buffer at pH 7.2.
Reprodcued from [32].
Reprodcued from [32].
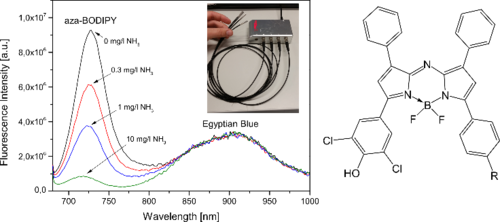
Figure 4.4. Response of the NH3-sensor based on aza-BODIPY dye and reference Egyptian blue phosphor. The insert shows the fiber optic sensors together with the read-out device from Pyro Science. Reprodcued from [33].