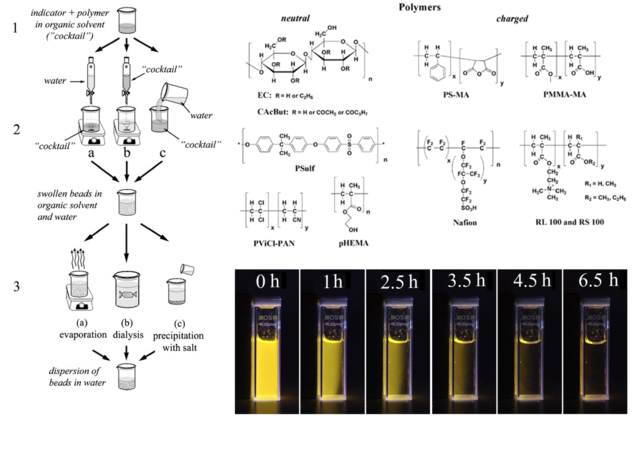
Figure 8.1. Left panel: illustration of the procedure for preparation of optical nanosensors via nanoprecipitation; upper right panel: chemical structures of the investigated polymers; lower right panel: photographic images of aqueous dispersion of magnetic oxygen sensitive nanoparticles under UV excitation during collection with a magnet. Reproduced from ref. [50].
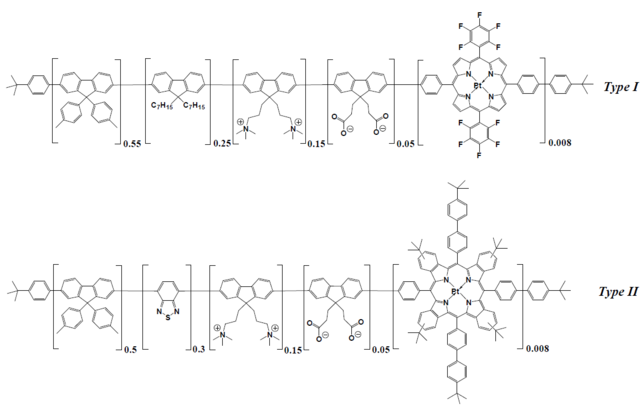
Figure 8.2. Chemical structures of the conjugated polymers used for preparation of oxygen nanosensors for 1-P and 2-P imaging. Reproduced from ref. [54].
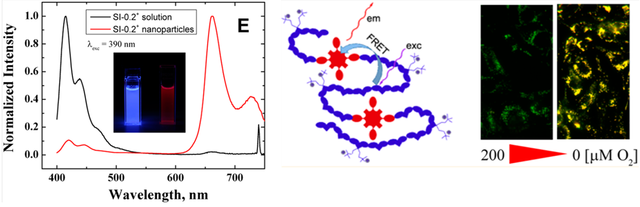
Figure 8.3. Left: emission spectra of the conjugated polymer dissolved in tetrahydrofuran and in the form of the nanoparticle dispersion in water illustrating efficient energy transfer from the conjugated polymer backbone to the oxygen indicator (middle); example of ratiometric intracellular measurement.