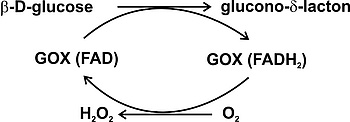
Figure 2.1. Mechanism of enzymatic oxidation of glucose. Reproduced from ref. [17,18].
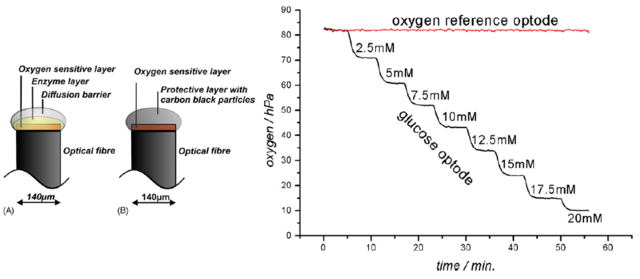
Figure 2.2. Glucose microoptodes for continuous glucose monitoring in subcutaneous tissue: left – schematic representation of the optode structure, right – example of the calibration curve. Reproduced from ref. [17,18].
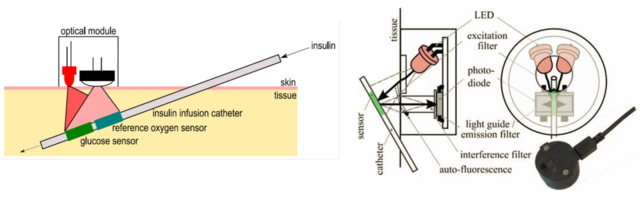
Figure 2.3. Schematic representation of the catheter-based subcutaneous glucose sensor (left) and the components of the read-out device designed for interrogation of glucose and oxygen sensors (right). Reprodcued from [19].