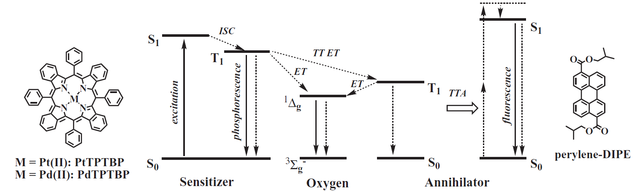
Figure 1.1. Mechanism of the TTA-upconversion exemplified for a metalloporphyrin and a perylene used as sensitizer and annihilator, respectively. Reproduced from ref. [3].
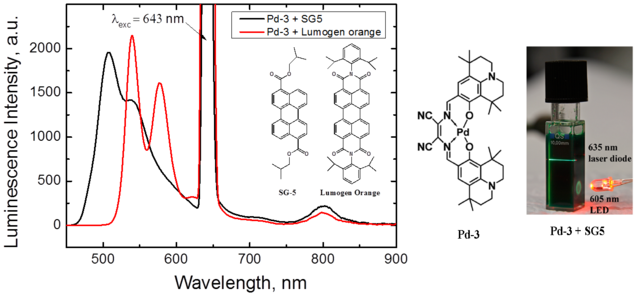
Figure 1.2. Upconversion spectra upon excitation with red light of the toluene solutions containing a mixture of the Pd(II)-Schiff base complex and a perylene dye; a photographic image of the same solution (SG-5 as annihilator) upon excitation with 635 nm laser diode and 605 nm LED. Reproduced from ref. [4].
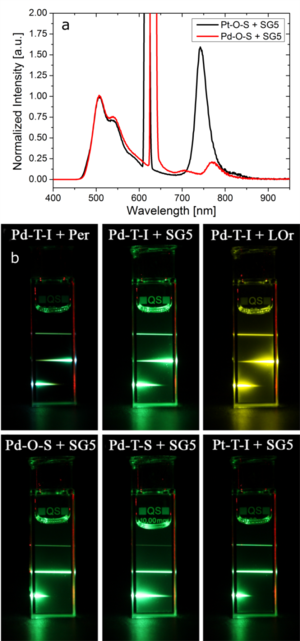
Figure 1.3. (a) Emission spectra of 1×10-4 M Pt/Pd-O-S complexes in toluene in presence of 5×10-4 M Solvent Green 5 (SG5) as annihilator when excited with a 450 W xenon-lamp. (b) Photographic images of 5×10-5 M deoxygenated toluene solutions of various sensitizers and different annihilators (C = 2.5×10-4 M; Per = perylene, SG5 = solvent green 5 & LOr = Lumogen orange) upon excitation with several laser diodes (675 nm, 650 nm and 635 nm, top to bottom).