Gas Chromatography with Conventional Detection
Gas chromatography is a separation technique which allows to separate and identify substances which can be volatilized without decomposition. This can range from permanent gases to substances with high boiling points (eg. up to n-Hectane C100H202 with a boiling point of 715°C).
Separation is done on GC columns with two different separation principles.
Gas Solid Chromatography (GSC) uses particles (activated charcoal, molecular sieves, alumina oxide) as stationary phases and the separation is based on adsorption. This technique is mainly used for low boiling substances and permanent gases. It is used only for special applications.
Gas Liquid Chromatography (GLC) uses liquid polymer coatings as stationary phases, separation is based on absorption (partition between the mobile and the stationary phase) is widely used in different application areas like
- environmental
- food and beverage
- clinical
- forensic
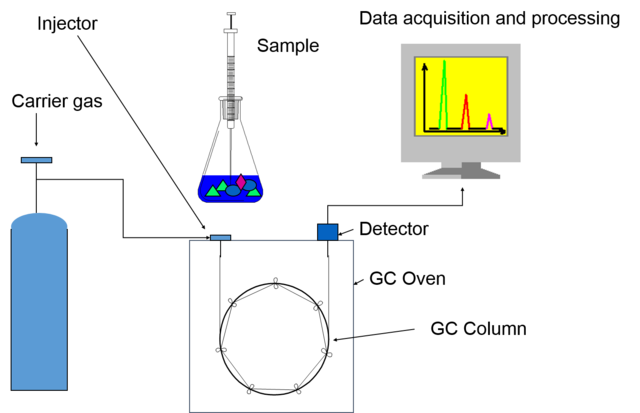
The mobile phase is the carrier gas (Helium, Nitrogen or Hydrogen) which pushes the analytes through the column.
Normally the sample is injected as a liquid into the inlet system and after the separation the analytes reach the detector where the properties of the analyses are processed and converted in an electric signal.
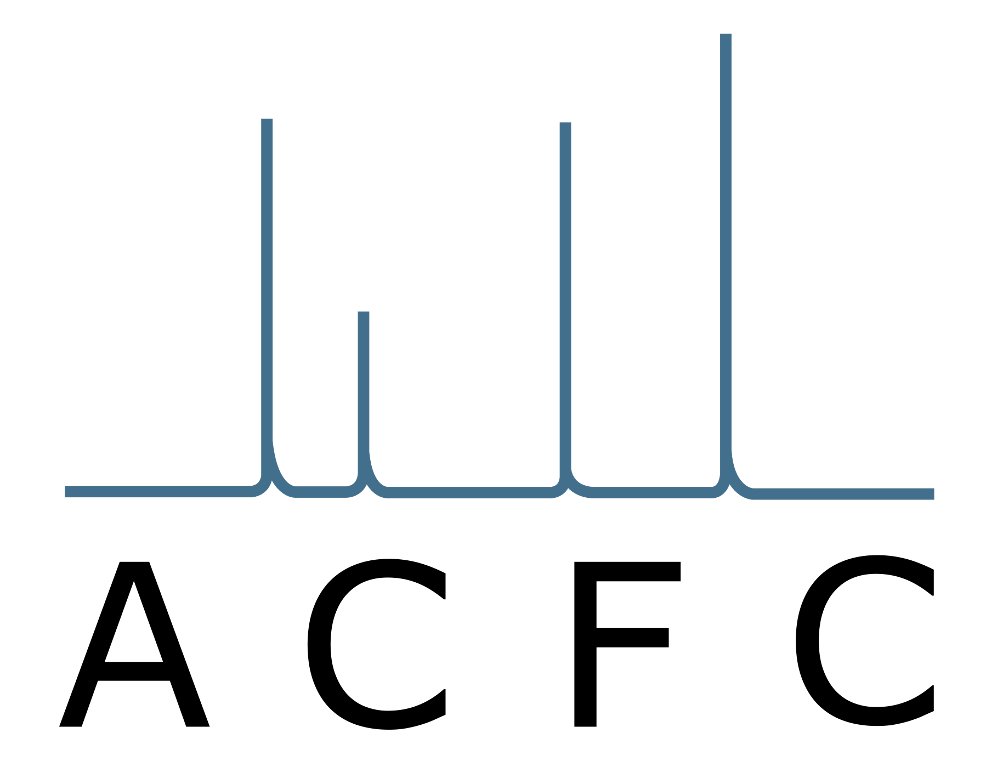
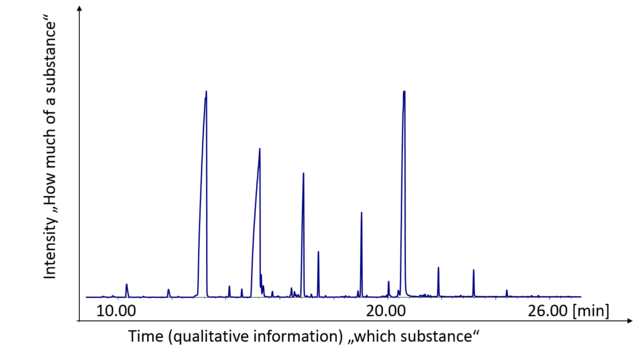
A chromatogram is displayed in Figure 2. Under constant parameters (carrier gas, stationary phase, column dimensions, temperature) one specific substance always elutes at the same retention time, so this is the qualitative information which allows an identification of an unknown substance (by comparison with pure reference substances). In addition, the peak area or the peak height correlates with the concentration. So, under ideal conditions you are able to get the information WHICH substances and at what CONCENTRATION they are present in your sample.
There are several detectors available, providing different information. In general, you can differentiate between universal and selective detectors. Universal detectors (by definition) see ALL the substances in your sample, while a selective detector just see specific properties of a molecule (eg. sulphur selective).
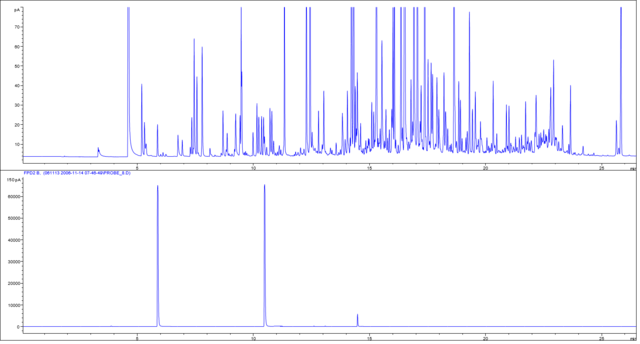
Figure 3. gives the example of a dual detection (after the separation the column flow is divided and transferred to an FID (universal) and flame photometric detector in the sulphur mode (FPD; selective). The sample displayed is a plant hydro distillate. With the universal detector you can detect more than hundred volatiles while the sulphur selective detector only “sees” three compounds which contain sulphur.