Mechanisms of carbonate mineral formation – Experimental approaches
Almost 20% of Phanerozoic sedimentary rocks are comprised of carbonate minerals. Most of our knowledge of past climatic conditions stems from the analyses of trace elements and stable isotopes in carbonate minerals. The main focus of the group of Mineralogy and Hydrogeochemistry of TU Graz is to mechanistically describe the physicochemical parameters controlling
- the chemical distribution of traces/impurities in carbonates and
- the stable isotope composition of major and trace elements in carbonates.
Specific tasks can be seen in the following sections.
Incorporation of trace elements and isotopic fractionation during carbonate formation
Contact: Martin Dietzel
Transformation behavior of amorphous calcium carbonate
Carbonates are widely distributed in marine and terrestrial environments and have been extensively studied in regard to reconstruct Earth’s system evolution. The occurrence and formation of calcium carbonate minerals is in most cases related to precipitation from aqueous solution where mineral growth proceeds along different pathways. The formation of ACC and its transformation to crystalline calcium carbonate (e.g. (Mg-)calcite, aragonite) is a key process in the formation of many biominerals.
To reconstruct formation conditions and crystallization pathways of those minerals the application of elemental, isotopic and structural proxies is a powerful tool. In particular, this requires detailed understanding on the distribution behavior of elements and isotopes during the formation and transformation of ACC to the crystalline phase. Therefore, ACC transformation experiments were carried out under precisely defined physicochemical conditions and in the presence or absence of organic and inorganic molecules.
Contact: Martin Dietzel
Formation Mechanisms of Carbonates via Amorphous Precursors
Recent studies focused on mineral growth from solutions supersaturated with respect to carbonates have shown that mineral formation does not always follow classic crystallization pathways, but proceeds via the formation of an intermediate amorphous phase. However, the mechanisms involved in this (trans-)formation are insufficiently understood and the chemical and isotopic evolution of fluids and solid counterparts during crystallization of amorphous precursors is, to date not explored at all. This project aims at investigating the reaction mechanisms controlling the formation of hydrous and anhydrous crystalline carbonates via amorphous precursors using well-established experimental set-ups.
Main focus of this project will be given on the formation behavior of calcium magnesium carbonates (CaxMg(1-x)CO3.nH2O) and calcium strontium carbonates (CaxSr(1-x)CO3). The innovative character of this project is that mineralogical observations, together with chemical and isotopic results, will be used to identify and describe formation mechanisms of crystalline carbonates via amorphous precursors. In this context, the fate of primary chemical and isotopic composition after the transformation of amorphous precursors to crystalline carbonate minerals will be assessed at high temporal resolution.
Contact: Martin Dietzel
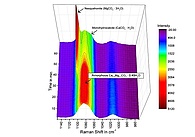
Figure: 3D Surface Plot of in situ Raman spectra showing the transformation behavior of amorphous Ca0.9Mg0.1CO3 0.49H2O dispersed in a MgCl2-NaHCO3 solution.
ACC formation in the scope of tailored transformation
Amorphous calcium carbonate is an important precursor for biomineralization, but highly metastable with transformation to crystalline polymorphs of CaCO3 between minutes and few days, depending on synthesis and storage conditions. Therefore characterising the amorphous precursor phase and gathering information about parameters governing crystallization is challenging with respect to monitor this highly metastable phase respectively the fast transformation process.
The aim of this study is to find synthesis routes on how to obtain long-time metastable amorphous calcium carbonate and subsequently be able to decipher crystallization pathways leading to the respected calcium carbonate polymorphs.
Contact: Martin Dietzel
Calibration of elemental and isotope proxies by inorganic precipitation experiment
The main task of this study is to develop proper proxy tools for the reconstruction of paleo-environmental conditions during the formation of biogenically induced carbonates. On that account the study focuses on the element partitioning and isotope fractionation processes in inorganic calcium carbonates precipitated under controlled laboratory conditions with and/or without the presence of selected organic substances.
The carbonate formation will be analyzed by using mineralogical and microstructural methods as well as elemental ratios, traditional and non-traditional stable isotopes, clumped isotopes and redox-sensitive elements. (Brachiopods as sensitive tracers of global marine Environment: Insights from alkaline, alkaline Earth metal, and metalloid trace element ratios and isotope systems)
Contact: Martin Dietzel
Oxygen and clumped isotope fractionation during carbonate mineral formation
Contact: Martin Dietzel
Crystallization of carbonates and sulfides from aqueous solutions using CO2 diffusion technique
Contact: Martin Dietzel