Alexander Kaufmann, Lukas Maier, Marlene Kienberger
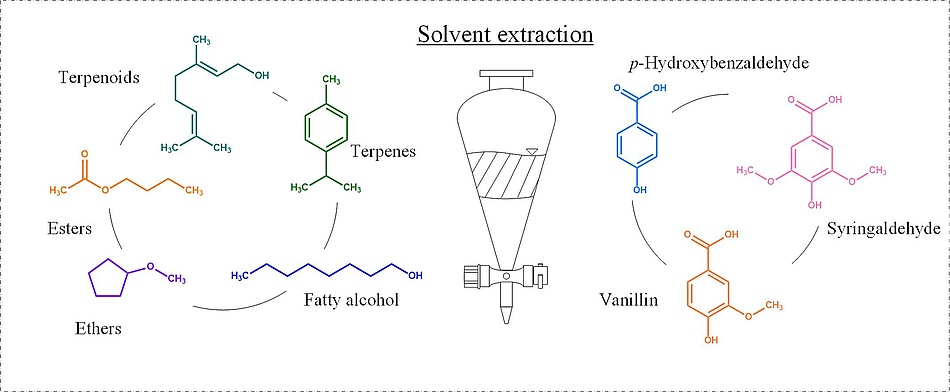
The aromatic aldehydes vanillin, syringaldehyde, and p-hydroxybenzaldehyde find applicability in a broad field ranging from additives in food, cosmetics, and fragrances to precursors of electrochemically active substances used in organic redox flow batteries. These aldehydes can be obtained by wet oxidation of lignin a macromolecule present in woody biomass followed by the isolation via solvent extraction from the resulting acidic solutions.
The present study investigates solvents grouped by esters, ethers, terpenes, and terpenoids, in addition to 1-octanol for their performance in extraction of the aromatic aldehydes vanillin, p-hydroxybenzaldehyde and syringaldehyde from an acidic aqueous solution. Solvent extractions are performed at 25 and 50 °C in double-walled tempered separation funnels. The overall extraction efficiency is determined by back-extractions performing a pH-swing at 25 °C. Aldehyde concentrations are measured by HPLC-UV. Karl-Fischer titration and total organic carbon measurements are used to account for the mutual solubility.
The best-performing solvent in the case of aldehyde yields at low mutual solubility is given by the ester butyl acetate with extraction efficiencies of 96.53 % for p-hydroxybenzaldehyde, and > 95 % and > 93 % for vanillin and syringaldehyde, respectively. The highest overall extraction efficiency among all solvents suitable for alkaline back-extraction is achieved by cyclopentyl methyl ether with 94.73 % for p-hydroxybenzaldehyde, 94.04 % for vanillin and 87.91 % for syringaldehyde. The solvents geraniol, 1-octanol, ethyl acetate, and 2-methyl tetrahydrofuran show increased uptake of water with ethyl acetate, and 2-methyl tetrahydrofuran pointing out a high solvent loss with 9.82 vol% and 15.84 vol%, respectively. The terpene p-cymene showed preferable extraction of vanillin and syringaldehyde over p-hydroxybenzaldehyde with mutual solubility below 0.02 vol%, which makes it and interesting candidate for multistage solvent extraction.
Separation and Purification Technology
Volume 340, 2024