Organic Tandem Solar Cells
Organic Tandem Solar Cells
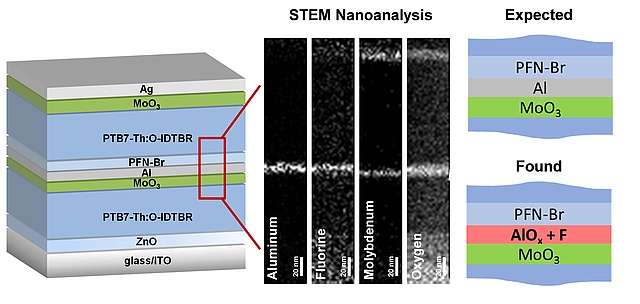
An unexpected phenomenon was found by Sebastian Höfler et al. in collaboration with the Institute of Electron Microscopy and Nanoanalysis and the Fondazione Bruno Kessler. STEM-EELS based elemental mappings and SIMS depth profiling reveal an accumulation of fluorine in the alumina containing recombination layer of the investigated tandem solar cells. This originates very likely from alumina−aryl fluoride interactions responsible for a partial defluorination of the conjugated polymer in the absorber layer. Since fluorinated absorber materials are commonly employed together with low work function metals like aluminum as electrode material or as recombination layer in organic photovoltaics, this alumina-aryl fluoride interaction is of high importance for organic electronic devices. These results have been published in Advanced Materials Interfaces
 Phase Separation in Nonfullerene Organic Solar Cells
Phase Separation in Nonfullerene Organic Solar Cells
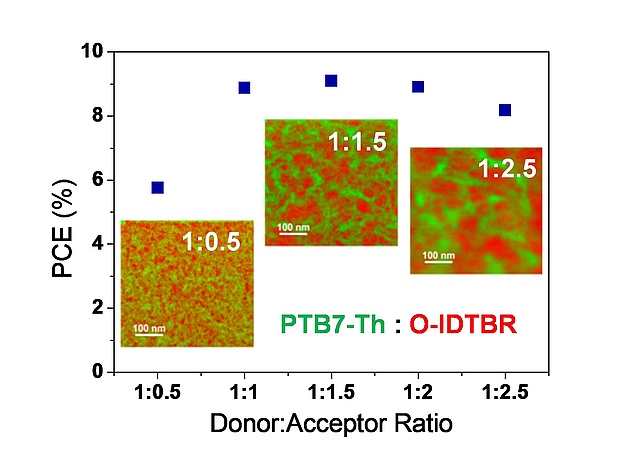
The performance of bulk-heterojunction organic solar cells strongly depends on the phase separation and domain sizes in the absorber layer. However, so far, only little is known about the phase separation in polymer:nonfullerene acceptor organic solar cells because of the chemical similarity of both phases, which makes the analysis of the phase separation difficult. In a collaborative study of the Trimmel group together with the Institute of Electron Microscopy and Nanoanalysis, the University of Nova Gorica and Anton Paar GmbH, the phase separation in the absorber layer of these solar cells was visualized in great detail by transmission electron microscopy elemental mapping using a direct electron detection camera. Differences in the phase separation could be well correlated to the solar cell performance and charge carrier mobilities. The paper has been recently accepted in ACS Applied Energy Materials
 New Christian Doppler-lab opened
New Christian Doppler-lab opened
On Sept. 24, 2019 the official opening of the CD-laboratory for Organocatalysis in Polymerization took place. In the presence of the rectorate, representatives of the Christian Doppler Society and the company partners Allnex Austria GmbH and Hilti AG the go-ahead for this project with a maximum duration of seven years was given.
The focus of this CD lab is on the cost-effective and environmentally friendly production of high-quality plastics, which are used, for example, for bonding, coating or protection against corrosion. Together with the company partners, Prof. C. Slugovc and his team are looking for ways to replace toxic and foul-smelling sulphur or nitrogen compounds as well as toxic metal compounds currently used in plastics production with more readily available, cheaper and less toxic alcohols. So far, such experiments have failed due to the low reactivity of alcohols, which – unlike thiols or amines – only react at high temperatures. A potential way of allowing alcohols to react under milder conditions is by organocatalysis. In this method, organic molecules are used to accelerate chemical reactions. To this end, the CD lab is now advancing the basics and knowledge of the activation of alcohols, developing tailor-made organocatalysts and investigating the properties of alcohol-based plastics in fastening technology and corrosion protection.
 2D NMR exchange spectroscopy on metastable (Pb,Ca)F2
2D NMR exchange spectroscopy on metastable (Pb,Ca)F2
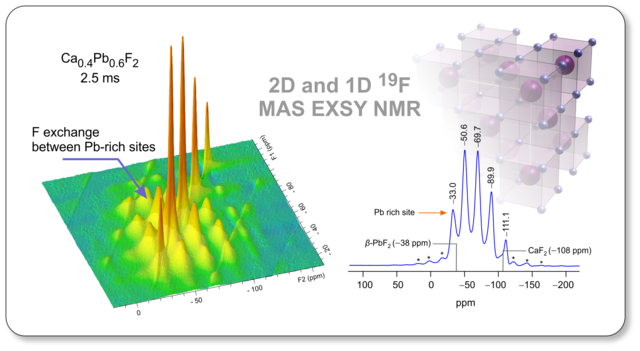
Mechanochemcally synthesized non-equilibrium phases represent ideal model compounds to study the effect of lattice disorder on F anion diffusivity and exchange processes in nanocrystalline materials. Sarah Lunghammer's study has just been accepted by Solid State Ionics and can be downloaded here.
 in operando magnetometry of battery cathode materials
in operando magnetometry of battery cathode materials
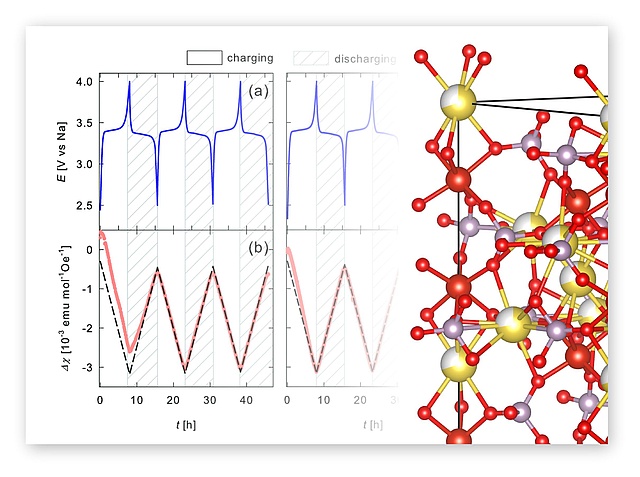
In a great NAWI collaboration together with the Würschum group at TU Graz, in operando magnetometry was used to study the redox processes in NVP (NaxV2(PO4)3). Roman Zettl and Ilie Hanzu synthesized NVP and helped carrying out the electrochemical experiments and interpreting the results. The paper has recently been accepcted by Phys. Chem. Chem. Phys.
 Li diffusion and ionic conduction in LLZO single crystals
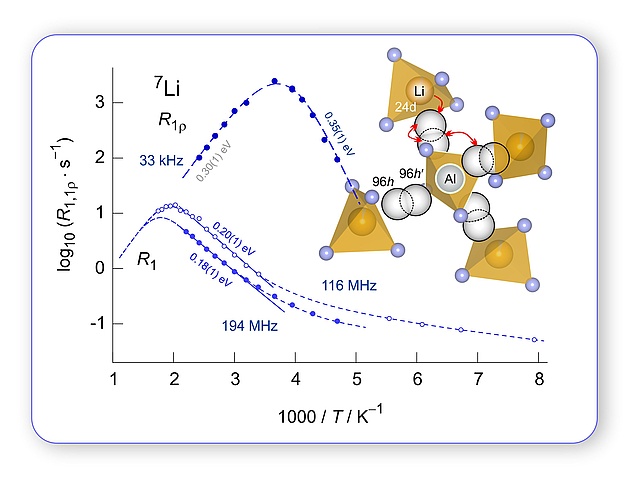
Li diffusion and ionic conduction in LLZO single crystals
Ceramic Electrolytes: sometimes Li(7) NMR senses more than (macroscopic) conductivity spectroscopy; first NMR spin-lattice relaxation study on Czochralski-grown Al-stabilized LLZO single crystals, great collaboration with IKZ Berlin. In his latest paper Patrick Posch showed how chemical inhomogenities affect spin-lock NMR rates that are sensitive to local and long-range Li hopping processes in LLZO-type garnets. Stil under debate how many elemantary diffusion processes ar at work in LLZO, which belongs to the most promising oxidic electrolytes for all-solid-state Li batteries.
 A solid with liquid properties: new class of electrolytes for all-solid-state batteries
A solid with liquid properties: new class of electrolytes for all-solid-state batteries
Replacing liquid electrolytes with a powerful solid would be a major breakthrough for all-solid-state batteries. In the new superionic conductor, studied by G. Hautier and Yuki Kato (Toyota Motor Europe) in collaboration with the Wilkening group, the very fast Li diffusion comes from a unique crystal structure which offers no strongly energetically favoured lattice sites leading to a so-called frustrated energy landscape: the ions are never "satisfied" where they sit. In this sense the ionic diffusion properties resemble those of a liquid. This specific diffusion mechanism could inspire the search for further materials offering the same feature. NMR helped us to characterize the two dynamic processes predicted by theory: the fast inter-ring and the even faster intra-ring Li ion hopping process.
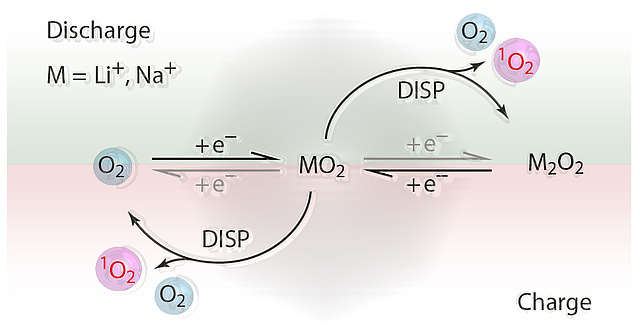
 Mechanism of singlet oxygen formation revealed - a major step for understanding metal oxygen batteries
Mechanism of singlet oxygen formation revealed - a major step for understanding metal oxygen batteries
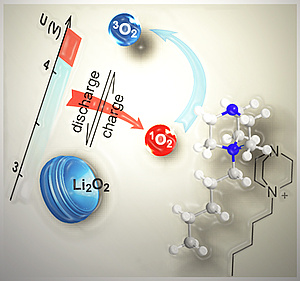
 Decarbonizing the energy system requires energy storage with large capacity but equally low economic and ecological footprint. Alkali metal-O2 batteries are considered outstanding candidates in this respect. However, they suffer from poor cycle life as a result of cathode degradation. Formation of the highly reactive singlet oxygen has been proposed to cause this degradation, but formation mechanisms have remained unclear. Here, Freunberger at al. have shown that the singlet oxygen source is the disproportionation of thermodynamically instable superoxide intermediates to the peroxides.
Decarbonizing the energy system requires energy storage with large capacity but equally low economic and ecological footprint. Alkali metal-O2 batteries are considered outstanding candidates in this respect. However, they suffer from poor cycle life as a result of cathode degradation. Formation of the highly reactive singlet oxygen has been proposed to cause this degradation, but formation mechanisms have remained unclear. Here, Freunberger at al. have shown that the singlet oxygen source is the disproportionation of thermodynamically instable superoxide intermediates to the peroxides.
The revealed mechanism conclusively explains the strongly growing degree of degradation when going from K-O2 to Na-O2 and Li-O2 cells. A major consequence is that highly reversible cell operation of Li-O2 and Na-O2 cells requires them to form and decompose the peroxides without disproportionation. Achieving this requires finding new reaction routes. The publication lays the mechanistic foundation to fight singlet oxygen as the predominant source of degradation in metal-O2 cells.
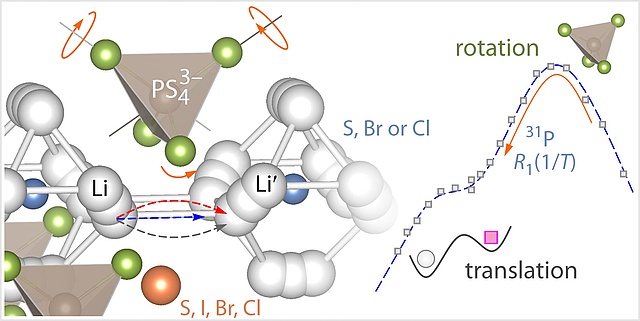
 Rotations vs. Hopping Processes: Holy Grails in Chemistry
Rotations vs. Hopping Processes: Holy Grails in Chemistry
Ion dynamics in argyrodite-type solid-electrolytes was explored by 31P NMR spin-lattice relaxation. Far from being understood completely how rotational dynamics influence translational Li movements and vice versa. Look at our latest findings published in Chem. Mater.

 Networking Event Energy Materials
Networking Event Energy Materials
On June 27th, 2019 a networking meeting on energy materials research at TU Graz took place (8:30 to about 18:00 in the lecture room H “Ulrich Santner” Kopernikusgasse 24).
The event was jointly organized by VARTA Micro Innovation GmbH and the lead-project Porous Materials @ Work and aimed at connecting the expertise of the many groups active in the field. Additionally, representatives of industries were present. The meeting consisted of talks (12+3 min) introducing the research fields, expertise and special equipment of the research groups, a poster session and ample time for networking.
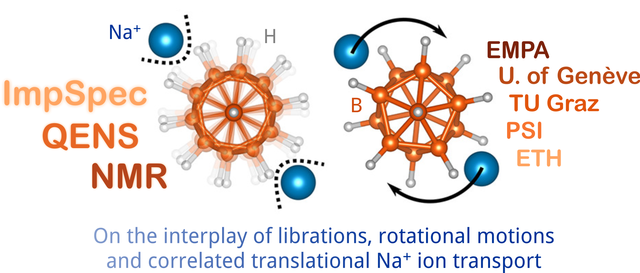
 Ion dynamics in closo-borates
Ion dynamics in closo-borates
Ionic conduction mechanism in closo-borates for sodium ion batteries elucidated. A portfolio of methods (NMR, QENS, ESI) was needed to see the full picture. Great collaboration with EMPA, Université de Genève, PSI, and ETH. Link: paper Chem. Mater.
 Electrochemistry of arylsilanes
Electrochemistry of arylsilanes
Electrochemical properties of arlysilanes - new paper in Electrochemistry Communications; Hanzu and co-workers throw light on the fascinating properties of 11 arylsilanes, 2 of them synthesized for the first time. Link: paper EC
 Ion dynamics in argyrodite-type thiophosphates
Ion dynamics in argyrodite-type thiophosphates
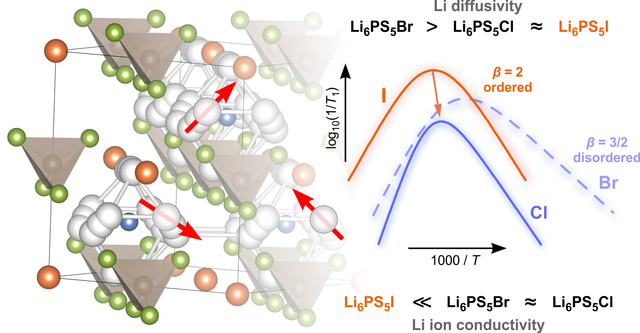
Li-bearing argyrodites play an important role as solid electrolytes for next-generation batteries. In a new publication, which appeared in RSC's PCCP, we report in detail on the complex interplay of local structures and ion dynamics. Importantly, also for the poor ion conductor Li6PS5I we found rapid ion dynamics on a local lenght scale. Link: paper in PCCP
 Ion dynamics in nanoglasses
Ion dynamics in nanoglasses
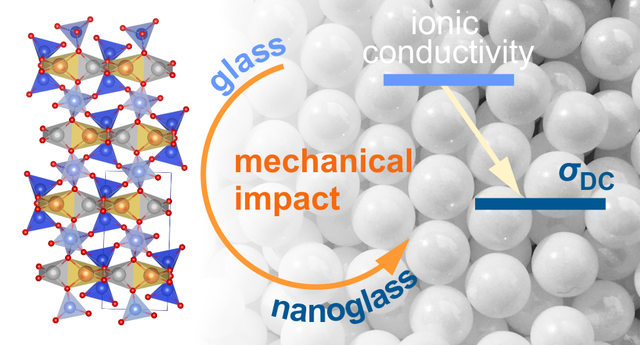
Li ion diffusivity is, in general, faster in glassy materials than in the crystalline counterparts. In particular, this relation holds for compounds with the same chemical composition. If a glassy sample is mechanically treated in a high-energy ball mill a material is obtained with a lower diffusivity as compared to that in the original sample. Obviously, structural relaxation is responsible for the decrease observed. Via modulus spectroscopy we followed the change in electrical relaxation and identified two distinct relaxation rates which we attribute to the situation in the unmilled glass and to Li species being already influenced by mechanical treatment. Link: paper in JPCC
 Singlet oxygen quenching in battery devices
Singlet oxygen quenching in battery devices
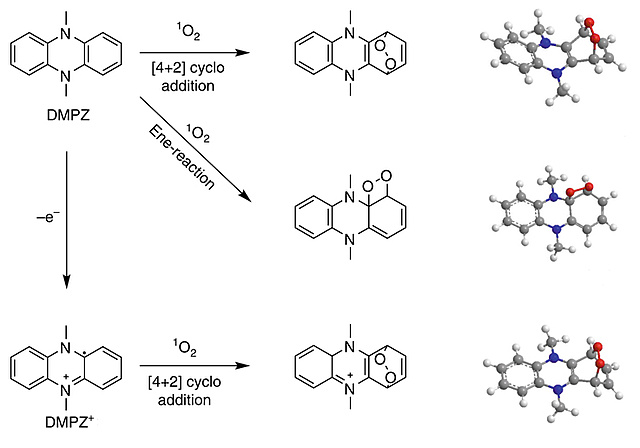
Singlet oxygen (1O2) causes a major fraction of parasitic chemistry during cycling of non-aqueous alkali metal-O2 batteries and also contributes to interfacial reactivity of transition-metal oxide intercalation compounds. Yet DABCOnium, the mono alkylated form of 1,4-diazabicyclo[2.2.2]octane (DABCO), is introduced in Angewandte Chemie as an efficient 1O2 quencher with an unusually high oxidative stability of ~4.2 V vs. Li/Li+. Previously known quenchers are strongly Lewis basic amines with too low oxidative stability. It is an ionic liquid, non-volatile, highly soluble in the electrolyte, and stable against superoxide and peroxide. The electrochemical stability covers the required range for metal-O2 batteries and greatly reduces 1O2 related parasitic chemistry as demonstrated for the Li-O2 cell.
 Singlet oxygen in Li-O2 batteries
Singlet oxygen in Li-O2 batteries
A joint undertaking of Korean, US American and Austrian researchers revealed singlet oxygen as the main reason for the gradual deactivation of redox mediators in lithium-oxygen batteries in an article in Nature Communications.
Non-aqueous lithium-oxygen batteries cycle by forming lithium peroxide during discharge and oxidizing it during recharge. The significant problem of oxidizing the solid insulating lithium peroxide can greatly be facilitated by incorporating redox mediators that shuttle electron-holes between the porous substrate and lithium peroxide. Redox mediator stability is thus key for energy efficiency, reversibility, and cycle life. It is shown that organic redox mediators are predominantly decomposed by singlet oxygen that forms during cycling. Their reaction with superoxide, previously assumed to mainly trigger their degradation, peroxide, and dioxygen, is orders of magnitude slower in comparison. Redox mediators must thus be designed for stability against singlet oxygen.
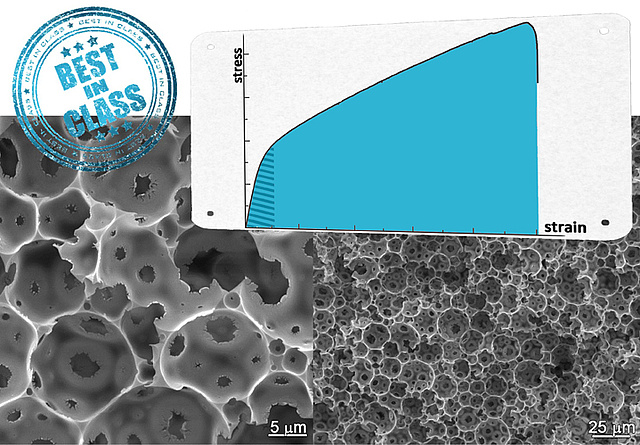
 Porous polymers - best mechanical properties in class
Porous polymers - best mechanical properties in class
Researchers of the National Institute of Chemistry, Ljubljana and ICTM underlined in a recent publication in Polymer the unique mechanical properties of emulsion templated macroporous polymer foams consisting of poly(dicyclopentadiene). The recent work deals with the influence of surfactant loading on strengths and toughness of this kind of porous matter which can be considered as the strongest and toughest macroporous polymer foams available up to now.
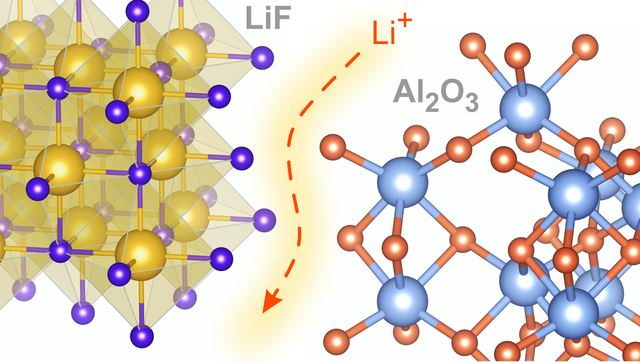
 Nanostructured Composites, new paper in JPCC
Nanostructured Composites, new paper in JPCC
Since the pioneering work of Liang in 1973 nano- or mesostructured composites consiting of an insulator and an ionically conducting phase act as model systems helpful to understand interfacial transport properties. (7)Li and (19)F NMR revealed details of the diffusion mechanism in LiF:alumina composites. Link to J. Phys. Chem. C
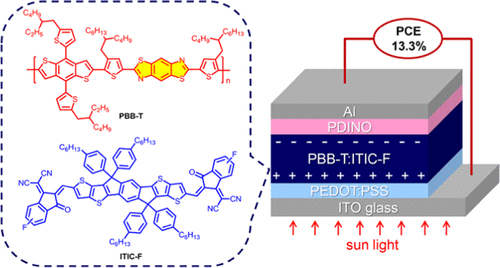
 A Benzobis(thiazole)-Based Copolymer for Highly Efficient Non-Fullerene Polymer Solar Cells
A Benzobis(thiazole)-Based Copolymer for Highly Efficient Non-Fullerene Polymer Solar Cells
In a collaborative publication of researchers of the Chinese Academy of Sciences, the South China Normal University, Austrian company Anton Paar GmbH and the Trimmel group non-fullerene polymer solar cells with a high efficiency of 13.3% were disclosed in Chemistry of Materials. The success is based on properly selecting and processing donor–acceptor pairs with suitable electronic properties and complementary optical absorption.
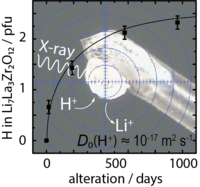
 Proton diffusion in cubic LLZO, new paper in JPCC
Proton diffusion in cubic LLZO, new paper in JPCC
A new paper has been published by the Rettenwander group about single-crystal X-ray diffraction to study the nature of Li+/H+ exchange in cubic Li7La3Zr2O12 (LLZO) garnets. On the basis of the proton concentration change over time (3 years), a spherical diffusion model was used to estimate the proton diffusion coefficient to be of the order of 2 × 10–17 m2/s. This diffusion coefficient is high enough to have a significant impact on both the cell performance and safety when LLZO is used as a protecting layer for Li metal anodes in Li-ion batteries that rely on liquid electrolytes. Link to JPCC.
 MSc/PhD positions available in the Wilkening/Hanzu group
MSc/PhD positions available in the Wilkening/Hanzu group
We seek to fill a PhD student position (30 h/week) in our research group in the course of March 2019. Topics: Solid-State Chemistry and Electrochemistry, new solid electrolytes, safety of all-solid-state batteries, spectroscopic methods (NMR, impedance spectroscopy, GPCL, GITT, etc.) You have a M.Sc. degree, Diploma or equivalent in Inorganic/Analytical Chemistry, Physical Chemistry or related areas, strong interest in Solid-State Physical Chemistry and application-oriented research. Contact: wilkening@tugraz.at or hanzu@tugraz.at
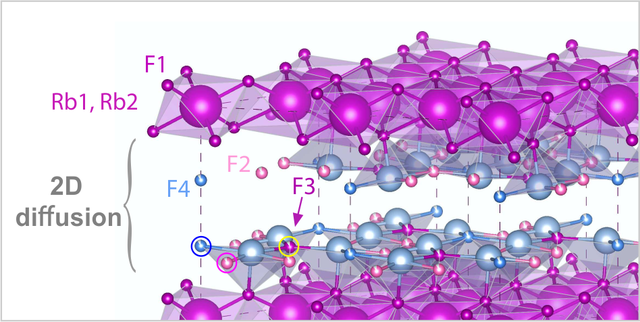
 2D Fluorine Diffusion, new paper in PCCP
2D Fluorine Diffusion, new paper in PCCP
A new paper about 2D F anion diffusion in nanocrystalline RbSn2F5 that has been prepared by a mechanochemical approach is out. We used broadband conductivity spectroscopy and nuclear magnetic relaxation to show that F preferably diffuses between the Rb-Sn layers. Analyzing the NMR relaxation rates with a spectral density function developed for 2D diffusion yields a consistent picture about F dynamics. This spectral density function has been introduced by P. M. Richards in 1978. Link to PCCP.
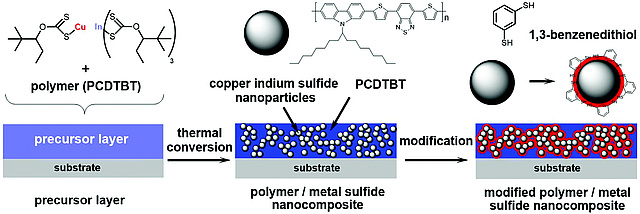
 Another new paper on CuInS2...
Another new paper on CuInS2...
...has been published by Thomas Rath et al. Dr. Rath assembled an interdisciplinary team for realizing his latest paper on ligand-free preparation of polymer/CuInS2 nanocrystal films and employing this strategy for improving the performance of solar cells. The work appeared as open access article in Journal of Materials Chemistry C.
 New paper on CuInS2 nanocrystals
New paper on CuInS2 nanocrystals
The Trimmel group realized an new synthesis route towards copper indium sulfide (CuInS2) nanocrystals with a size of 3–4 nm and a chalcopyrite crystal structure starting from copper and indium xanthates as precursors in a hot injection synthesis performed at a temperature of 200 °C and used the material as acceptor in polymer/nanocrystal bulk heterojunction solar cells. The work has been published in New Journal of Chemistry as an open access arcticle.
Understanding fast-ion conduction in solid electrolytes
16 – 17 March 2020
The Royal Society at Chicheley Hall: home of the Kavli Royal Society International Centre
royalsociety.org/science-events-and-lectures/2020/03/conduction-electrolytes