2. pH indicators
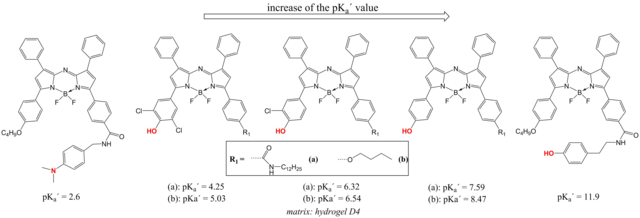
Several classes of fluorescent pH indicators have been investigated in our group. Aza-BODIPY dyes (Fig. 2.1) are particularly attractive due to efficient absorption in the red part of the spectrum (ε ~80,000 M-1cm-1), fairly bright NIR fluorescence and unmatched photostability. We prepared a palette of the aza-BODIPY indicators covering the whole pH range. [15,16] In most cases, the tetraaryl-aza-BODIPYs were modified with a hydroxyl-group to introduce the pH sensitivity. The phenol moiety barely affects the fluorescence of the chromophore in the protonated state, but switches its off when upon deprotonation due to Photoinduced Electron Transfer (PET). Simultaneously, a bathochromic shift of the absorption is observed, indicating a charge transfer character of the group. Electron-withdrawing substituents in o-position towards the OH-group significantly decrease the pKa values whereas the electron donating O-CH3 groups located in the other aryl substituents slightly increase the pKa values. The pKa values can be extended into highly acidic and highly basic region by introduction of aromatic amino-groups and phenol groups, respectively, operating solely via PET. [16] Mixing the indicators with extremely similar photophysical properties but different pKa values allowed preparation of a wide dynamic range pH sensor. [16]
Figure 2.1. Chemical structures and apparent pKa' values of the fluorescent aza-BODIPY indicators reproduced from [15,16].
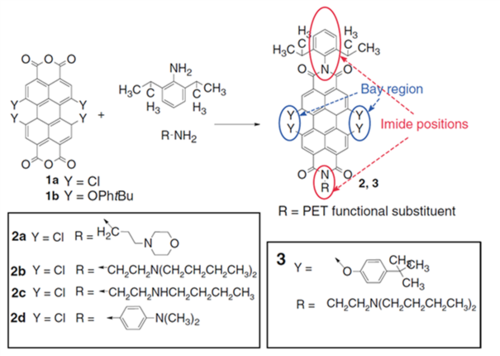
Perylenes belong to very bright, chemically and photochemically stable dyes which are applied in various fields of science and technology. They show absorption in the blue-yellow part of the spectrum depending or respective substituents. Perylene bis-imides were rendered pH-sensitive by incorporating dialkyl-amino-bearing moieties into imide positions of the perlylene (Fig. 2.2) [17] These groups are known to act as fluorescence quenchers operating via photoinduced electron transfer (PET): whereas the neutral form of the amine causes PET from the receptor to the dye, the protonated quaternary ammonium is not a quencher. The PET efficiency was dependent on the red-ox potential of the perylene: more electron-negative tetrachloro-derivatives (2a-d series) showed more efficient quenching than the tetra-t-Bu-phenoxy-modified dye (3). The pKa value could also be fine-tuned (e.g. 1.1, 5.2 and 6.5 for 2d, 2b and 2c, respectively). Based on the above concept, hydrophilic sulfonated derivatives were synthesized. [18] Incorporated into Rl-100 beads they allowed for sensing and imaging of pH via measurement of the fluorescence decay time.
Figure 2.2. Chemical structures of new pH probes based on perylene dyes reprodcued from [17].
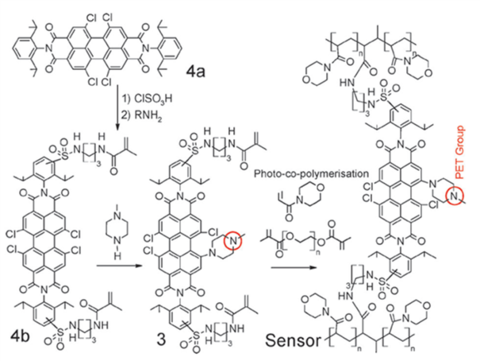
In order to overcome the risks of dye migration and leaching, a new perylene-based indicator was prepared and covalently immobilized into a hydrophilic hydrogel (Fig. 2.3). [19] Introduction of the electron-donating nitrogen into the bay position resulted into a significant bathochromic shift of the absorption and emission of the perylene, enabling excitation in the red part of the spectrum and detection of the emission in NIR. The second nitrogen of the piperazine group was responsible for the pH sensitivity via PET effect. Acrylamido groups for covalent coupling were introduced via chlorosulfonation of the aromatic moieties in the imide position. A new hydrogel was prepared via cross-linking of acryloylmorpholine using a UV photoinitiator and immobilizing the pH indicator into the network (Chart 13). Covalent immobilization was very helpful for avoiding the hysteresis effects and the sensor showed fast and reproducible response.
Figure 2.3. Synthesis and structures of the perylene-based NIR pH probe with indicator covalently embedded into a novel hydrogel matrix reprodcued from [19].
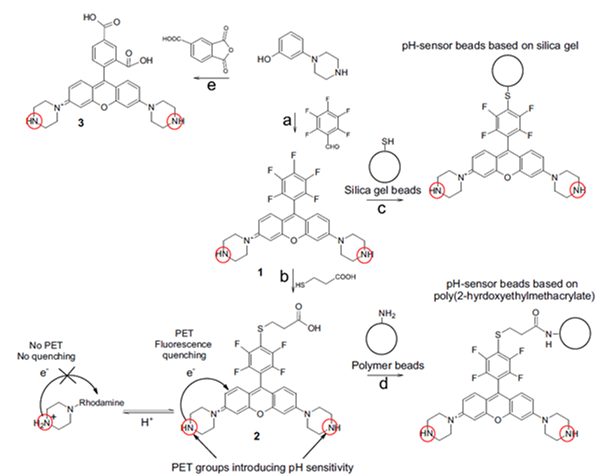
Other classes of indicators are of much interest since they can enable higher flexibility of photophysical properties, synthesis and further modification (e.g. covalent coupling). Therefore, new pH probes based on the rhodamine chromophore (Fig. 2.4) [20] contain piperazino groups responsible for pH-dependent PET quenching, whereas pentafluorophenyl-groups, enabled covalent coupling of the indicators via Click reactions with thiols. The dye was either directly coupled to the silica-gel beads modified with a thiol (route C) or first the carboxylic group was introduced to enable covalent coupling onto poly(hydroxyethylmetacrylate) (pHEMA) beads. Particularly, the new dyes could be rendered with a lipophilic tail via reaction with an aliphatic thiol or coupled to poly(hydroxyethylmethacrylate) poly(HEMA) beads. Very high molar absorption coefficients of the rhodamine (about 90,000 M-1 cm-1) in combination with good quantum yields (about 70 % for the protonated “on” form) make the new probes particularly attractive for practical applications.
Figure 2.4. Synthesis and structures of the novel rhodamine-based indicators and pH-sensitive microparticles based on covalently immobilized dyes reprodcued from [20].
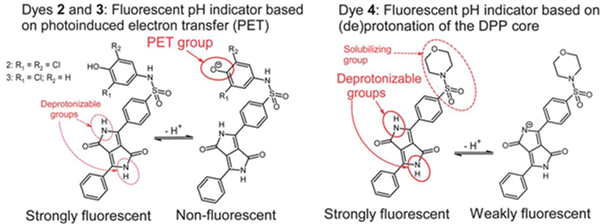
Diketopyrrolopyrrols (DPPs) represent highly stable and cheap pigments. We demonstrated that they can be modified in a simple way to result in pH indicators (Fig. 2.5) [21] Two pH sensing mechanisms could be distinguished: (i) deprotonation of the lactam nitrogens of the DPP leading to bathochromic shift of absorption (dyes 2-4) and emission (dye 4) and (ii) deprotonation of the phenol moiety in dyes 2 and 3 resulting in fluorescence quenching via PET (no change in the form of the absorption and emission spectra). Whereas the pKa value for the first process is rather high (9.75 for dye 4), it is much lower for monochlorophenol (pKa 7.63, dye 3) and dichlorophenol (pKa 6.49, dye 2) receptors. Unfortunately, photostability of the resulted indicators was not high.
Figure 2.5. Chemical structures and sensing mechanism of pH indicators based on diketo-pyrrolo-pyrrole dyes reprodcued from [21].
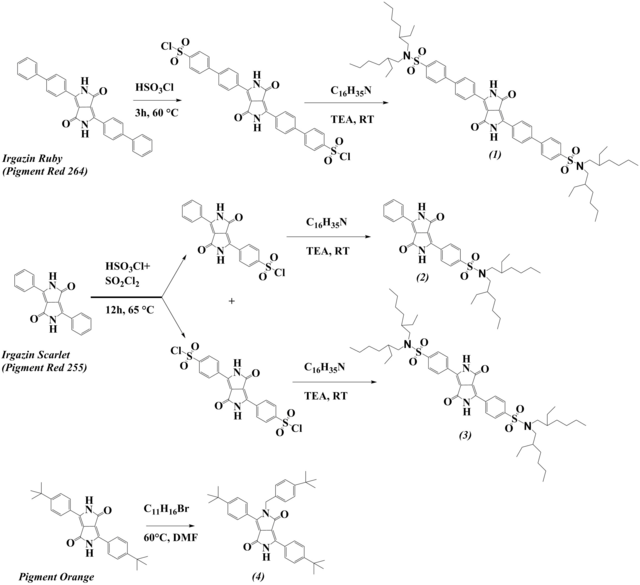
Fluorescent pH indicators are not only useful for preparation of pH optodes but also for design of optical sensors for acidic and basic gases. A new class of pH indicators for carbon dioxide sensors based on diketopyrrolopyrrol chromophore has been introduced via simple modification of the commercially available cheap pigments. [22] In the first work, chlorosulfonation of the phenyl rings and subsequent reaction with secondary amines resulted in lipophilic indicators (Fig. 2.6) excellently soluble in organic solvents and in polymers. Deprotonation of the lactam nitrogen results in significant bathochromic shift of the absorption and emission spectra. Importantly, it is possible to tune the pKa value of the indicator: di-substituted biphenyldiketopyrrolopyrrol (1) and mono-substituted phenyldiketopyrrolopyrrol (2) have the highest pKa (highest sensitivity of the CO2 sensors) whereas the di-substituted phenyldiketopyrrolopyrrol (3) has much lower pKa value and is suitable for preparation of less sensitive CO2 sensors. All the indicators possessed very bright emission of the protonated form (QYs close to 1, however only moderately intense emission for the deprotonated form (QYs ~10 %). Thus, in the following work we synthesized a new diketopyrrolopyrrol dye (4, Fig. 2.6) via a one-step alkylation of a commercially available pigment. [23] The dye shows good solubility in organic solvents and polymers and showed pH-dependent equilibrium due to deprotonation of the remaining lactam nitrogen. The emission spectra for the protonated and deprotonated forms showed excellent compatibility with the green and red channel, respectively, of an RGB camera thus making possible imaging of pCO2 with a compact and low-cost set-up. Notably, the fluorescence quantum yields were comparable for both forms of the dye (86 and 66 % for protonated and deprotonated forms, respectively). Unfortunately, the photostability of the new dyes is rather low making them not suitable for prolong measurements at high light intensity.
Figure 2.6. Synthesis and structures of novel diketopyrrolopyrrole-based pH indicators for carbon dioxide sensing reprodcued from [22,23].
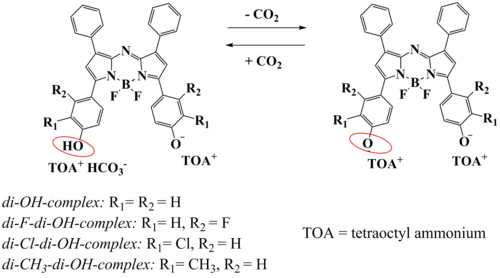
Novel dihydroxy-aza-BODIPY dyes (Fig. 2.7) [24] are not fluorescent but show pH-dependent absorption changes in NIR part of the spectrum making them suitable for preparation of optical carbon dioxide sensors. Equilibrium between the mono-deprotonated (in presence of CO2) and doubly deprotonated form (in the absence of CO2) is utilized. Both forms show significantly different NIR absorption (Fig. 2.7). The pKa values for the second deprotonation step were significantly higher than for the first one (e.g. 8.18 and 10.34, respectively, for di-OH-complex) which ensures higher sensitivity of some dye representatives. Importantly, the pKa values, and consequently the sensitivities, are tuneable over a broad range by varying the nature of the substituent (electron-withdrawing or electron-donating) located in ortho- or meta-position in respect to the hydroxyl-group. For instance, the highest pKa (second deprotonation step) is observed for di-CH3-di-OH complex (pKa 10.68) and the lowest for di-Cl-di-OH-complex (pKa 8.72). Although the new indicators are of colorimetric nature, they also have been used in a luminescence read-out based on inner-filter effect. [24]
Figure 2.7. Chemical structures and sensing mechanism of aza-BODIPY pH colorimetric indicators for sensing of carbon dioxide reprodcued from [24].