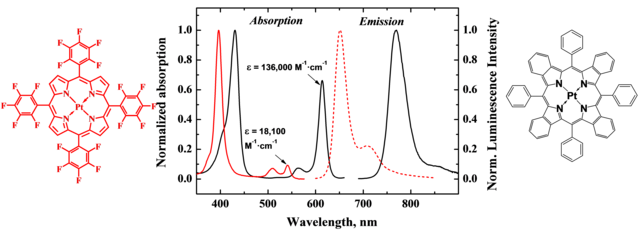
Figure 1.1. Spectral properties of the conventional porphyrin (left) and π-extended analogue (right) reproduced from [2].
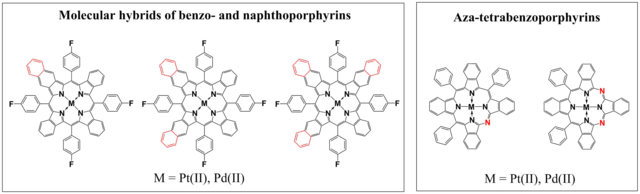
Figure 1.2. Chemical structures of molecular hybrids of benzo- and naphthoporphyrins and aza-tetrabenzoporphyrins reproduced from [3].
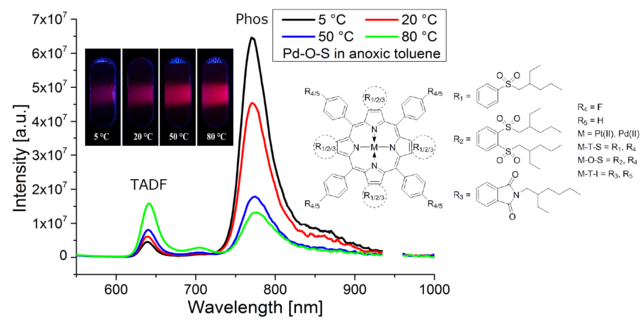
Figure 1.3. Chemical structures of new highly electron-deficient benzoporphyrins; temperature dependency of the emission spectra in anoxic toluene and the corresponding photographic images (365 nm excitation) for the Pd-O-S complex. Reproduced from [4].
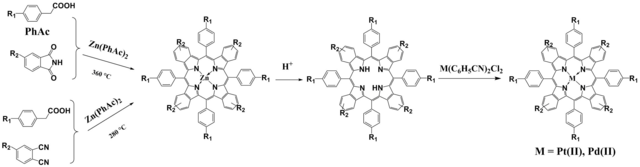
The benzoporphyrins are commonly prepared via the Lindsey method. [5] Briefly, the cycloxehenoporphyrins are prepared via condensation of tetrahydroisoindole with ethylisocyanoacetate, the resulted ligand is metalated and the cycloxeheno-rings finally oxidized to produce benzoporphyrin complexes. Although this method results in pure porphyrin products, it has some disadvantages due to the high cost of the precursors for tetrahydroisoindole preparation and the fact that only diluted solutions of the educts can be used in the condensation step, which makes upscaling of the synthesis challenging. In the alternative template method (Fig. 1.4) [6] zinc benzoporphyrin is prepared via high temperature template condensation of phthalimide with phenylacetic acid using zinc phenylacetate as a template, the zinc complex is demetallated in acidic media and Pt(II) or Pd(II) complexes are synthesized by boiling a metal precursor and the metal-free ligand in a non-coordinating solvent. We demonstrated that the template condensation can be achieved with good yields about 10 % from extremely cheap materials and enables synthesis of benzoporphyrin complexes on multi-gram scale (which is particularly valuable for potential applications in photovolataics and food packaging). Resulted porphyrins, unfortunately, contain a number of derivatives resulting from condensation of a benzyl group (originating from phenylacetic acid). We have shown that preparation of analytically pure complexes is possible via substitution of phthalimide for o-dicyanobenzene. [7]
Figure 1.4. Synthesis of benzoporphyrin complexes via a template condensation from phthalimides and via a modified template condensation via 1,2-dicyanobenzenes reproduced from [6].
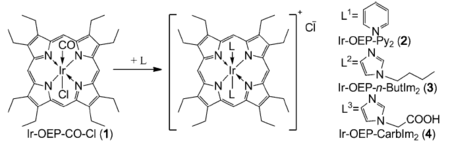
Pt(II) and Pd(II) porphyrin complexes possess square planar geometry so that synthetic modifications are only possible on macrocycle. Substitution of the central metal for Ir(III) allows for phosphorescent complexes of octahedral symmetry (Fig. 1.5). We showed that these dyes are viable oxygen indicators and substitution of the axial ligands allows tuning of solubility and photophysical properties. [8] The luminescence decay times of the complexes vary over a wide range (27-97 μs). We also demonstrated that these complexes can be used in combination with peptides (attached via axial ligands) as intracellular oxygen probes. [9]
Figure 1.5. Chemical structures of iridium(III) octaethylporphyrin complexes with various axial ligands reproduced from [8].
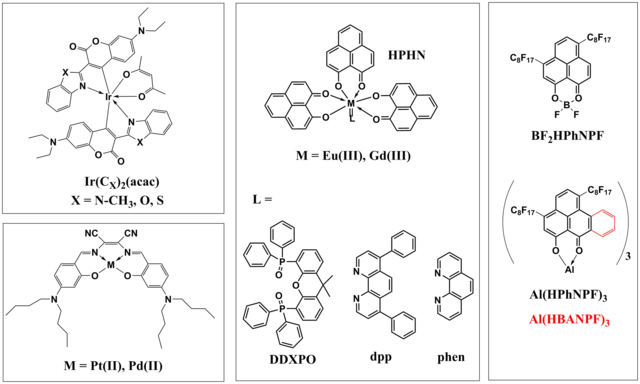
Other classes of luminescent dyes were also prepared and characterized in our group (Fig. 1.6). Easily accessible from fluorescent laser dyes, ultra-bright cyclometallated iridium(III) complexes [10] feature particularly high molar absorption coefficients (93,000 M-1cm-1) and very strong room temperature phosphorescence (QYs up to 54 %). The sensors on their basis were applied for oxygen imaging in biofilms. [11] The drawback of the iridium(III) coumarin complexes is their moderate photostability.
Figure 1.6. Chemical structures of other investigated indicators suitable for optical oxygen sensing; reprodcued from [10].
Pt(II) and Pd(II) complexes with donor-acceptor Schiff bases (Fig. 1.6) were synthesized via a very simple 2-step procedure. [12] The dyes possess very efficient absorption in the orange part of the spectrum (>110,000 M-1cm-1) and show moderately strong NIR phosphorescence (QYs ~10 % and 2 % for Pt(II) and Pd(II) complexes, respectively). These dyes were demonstrated to be promising as sensitizers for triplet-triplet annihilation-based upconversion.
Recently reported Eu(III) complexes with 8-hydrohyphenalenone (HPhN) (Fig. 1.6) [13] show very interesting photophysical properties. They possess characteristic narrow-band red emission of Eu(III) upon excitation with blue light (up to 475 nm). The luminescence quantum yields are about 20 % which is extraordinary high for such low-energy excitation. The most interesting fact, however, is that the emission of these dyes is highly sensitive to oxygen. In fact, the bimolecular quenching constant is similar to that of the conventional phosphorescent dyes. This can be explained by very the proximity of the triplet state of the antenna and 5D0 state of Eu(III) which promotes efficient back energy transfer to the ligand. Optical oxygen sensors based on the above complexes possess very narrow emission and can be particularly promising for microscopic applications.
In contrast to the Eu(III) chelates, gadolinium(III) complexes with the same ligand [13] show phosphorescence originating from triplet state of the HPhN ligand. The phosphorescence is very strong (QYs 42-56 %) which demonstrates that Gd(III) complexes can be efficient triplet emitters at room temperature. The phosphorescence decay times are fairly long (1.2 - 2.1 ms) which ensures high sensitivity to oxygen even in the polymers of moderate oxygen permeability such as polystyrene. A new class of phosphorescent dyes – BF2 and Al(III) chelates of 8-hydroxyphenalenon and the benzoannelated derivative HBAN [14] (Fig. 1.6) are excitable with blue light and show extraordinary long phosphorescence decay times (300-600 ms). Incorporation of these dyes in perfluorinated polymers results in ultra-trace oxygen sensors.
Recently reported Eu(III) complexes with 8-hydrohyphenalenone (HPhN) (Fig. 1.6) [13] show very interesting photophysical properties. They possess characteristic narrow-band red emission of Eu(III) upon excitation with blue light (up to 475 nm). The luminescence quantum yields are about 20 % which is extraordinary high for such low-energy excitation. The most interesting fact, however, is that the emission of these dyes is highly sensitive to oxygen. In fact, the bimolecular quenching constant is similar to that of the conventional phosphorescent dyes. This can be explained by very the proximity of the triplet state of the antenna and 5D0 state of Eu(III) which promotes efficient back energy transfer to the ligand. Optical oxygen sensors based on the above complexes possess very narrow emission and can be particularly promising for microscopic applications.
In contrast to the Eu(III) chelates, gadolinium(III) complexes with the same ligand [13] show phosphorescence originating from triplet state of the HPhN ligand. The phosphorescence is very strong (QYs 42-56 %) which demonstrates that Gd(III) complexes can be efficient triplet emitters at room temperature. The phosphorescence decay times are fairly long (1.2 - 2.1 ms) which ensures high sensitivity to oxygen even in the polymers of moderate oxygen permeability such as polystyrene. A new class of phosphorescent dyes – BF2 and Al(III) chelates of 8-hydroxyphenalenon and the benzoannelated derivative HBAN [14] (Fig. 1.6) are excitable with blue light and show extraordinary long phosphorescence decay times (300-600 ms). Incorporation of these dyes in perfluorinated polymers results in ultra-trace oxygen sensors.