3. Fluoroionophores
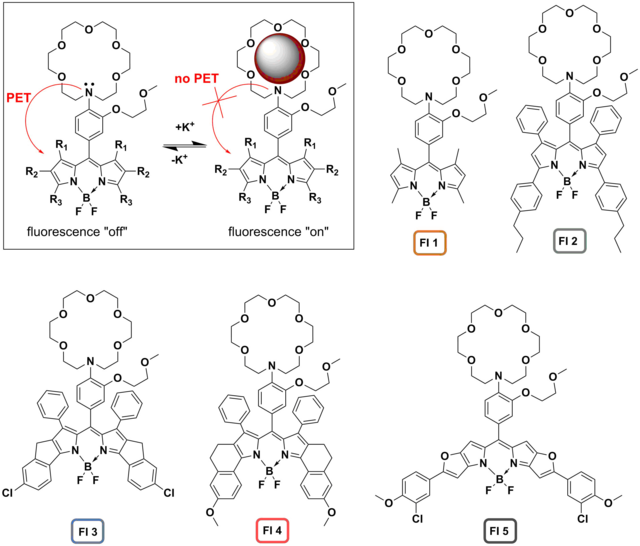
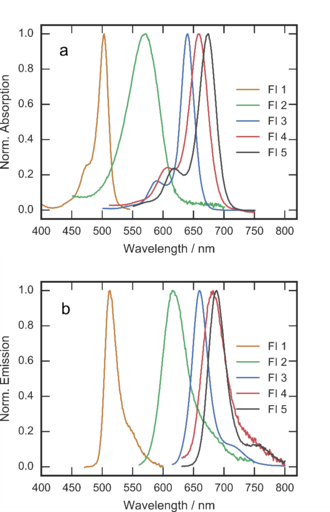
The research of the group is also dedicated to synthesis of fluoroionophores and their application in optical sensors for ions. Fluoroionophores represent a combination of a fluorescent dye (fluorophore) and an ion-sensitive receptor (ionophore). The receptor moiety not only allows for reversible coordination of the ion of interest but also enables modulation of the fluorescence of the fluorophore. In the absence of the analyte, the fluorescence is quenched due to photoinduced electron transfer (PET) from the nitrogen of the receptor to the chromophore. Upon coordination of the metal ion, inhibition of PET results in great enhancement of fluorescence. We prepared a palette of new fluoroionophores for potassium based on BODIPY chromophores and aromatic aza-18-crown-6 receptor bearing lariat side-group (Fig. 3.1). [25] The spectral properties of the dye are systematically tuned: whereas FI 1 shows absorption and emission in the green part of the spectrum (Fig. 3.2), other dyes absorb and emit efficiently in the red-NIR. All the fluoroionophores exhibit high brightness with quantum yields between 0.20 and 0.47 in the “on” state and a molar absorption coefficient between 30,000 and 290,000 M-1cm-1. Importantly, the aromatic receptor renders the dyes inert to pH changes at physiological conditions, since the protonation of the receptor is observed at pH < 5.5. The selectivity over sodium and other cations is very high, NH4+ being the only interfering species at physiological conditions.
Figure 3.1. Operating principle of a K+ fluoroionophores and chemical structures of the synthesized probes reprodcued from [25].
Figure 3.2. Normalized absorption (a) and emission (b) spectra of the fluoroionophores in dichloromethane. Reprodcued from [25].
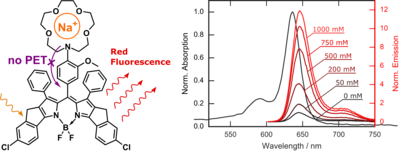
Substitution of the aza-18-crown-6 receptor with smaller of the same chromophore with smaller aza-15-crown-5 renders the fluoroionophores sensitive to sodium (Fig. 3.3). [26] Similarly to K+-fluoroionophores, the Na+ probes are inert to pH changes above pH 5.5 and show virtually no cross-talk to K+, Ca2+ and Mg2+. They were excellently suitable for preparation of optical sensors for monitoring seawater salinity.
Figure 3.3. Chemical structure and spectral properties of the Na+ fluoroionophore. Reprodcued from [26].