Project Leader
Heidrun Gruber-Wölfler
Assoc.Prof. Dipl.-Ing. Dr.techn.
Assoc.Prof. Dipl.-Ing. Dr.techn.
- Phone
- +43 316 873 - 30406
- Fax
- +43 316 873 - 1030406
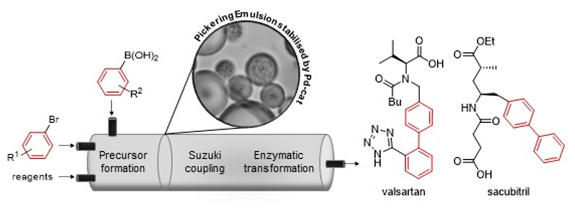
Hiebler, K., Gruber-Woelfler, H. et. al. (2018)
Beilstein Journal of Organic Chemistry, 14, 648–658.