Good brown fat to combat obesity
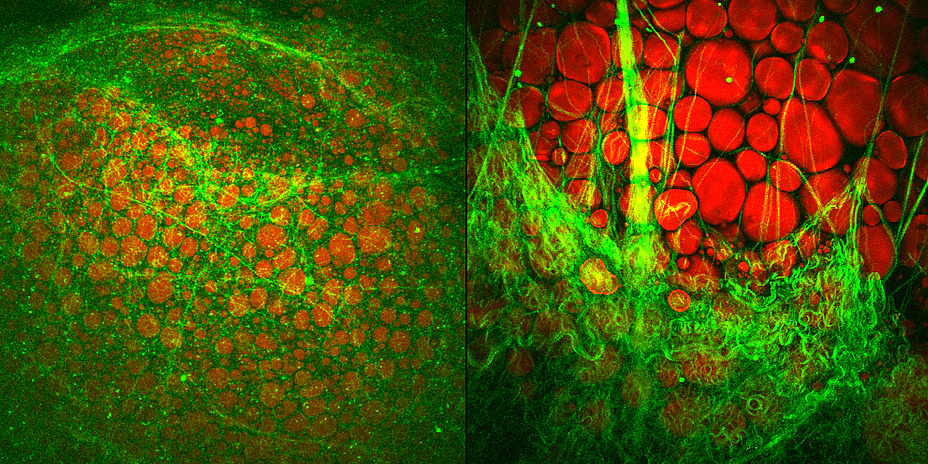
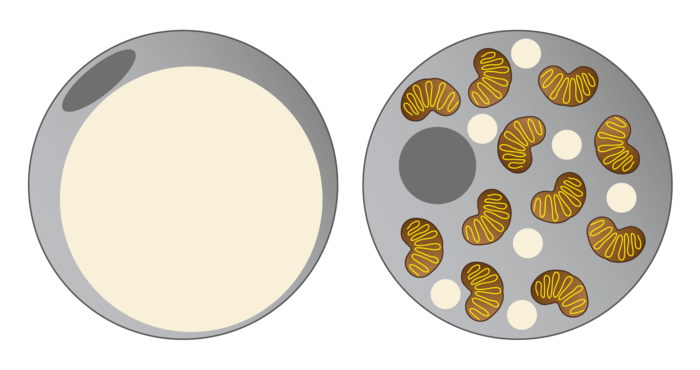
Brown fat cells examined molecularly
At the Institute of Biochemistry at TU Graz, the research group led by Juliane Bogner-Strauss are dedicating themselves to this subject at a molecular level. In a project funded by the Austrian Science Fund (FWF), the researchers want to discover what fundamental switching circuits and paths control the development of brown fat cells and their activity. As early as 2013 they were able to identify a protein – N-acetyltransferase 8-like (NAT8L) – in the mitochondria of brown fat cells which influences their activity.N-acetyl-aspartate: a molecule with a double mission
NAT8L is the enzyme which enables production of a particular amino acid, N-acetyl-aspartate (NAA), in mitochondria. "NAA was only recently known to be an essential intermediate product of lipid and cholesterol synthesis in the brain. We were able to show that NAA doesn’t only play an important role in the brain, but also controls the activity of brown fat cells," explains Juliane Bogner-Strauss. In a recently published study, the Bogner-Strauss team were able to show that not only production, but also the breakdown of NAA is responsible for increasing activity in brown fat cells. Because if NAA accumulates in brown fat cells and cannot be broken down, this surprisingly has a negative effect. "We’ve established in our in vitro experiments that increasing concentrations of NAA in brown fat cells lowers their metabolic activity. The real key role is played by an enzyme which actuates the breakdown of NAA in the cytosol of the fat cell," says Juliane Bogner-Strauss.If this enzyme which is called aspartoacylase is inhibited, less cytosolic acetyl-CoA is available to the cell, thus decreasing histone acetylation in the cell nucleus of the fat cell. Histones are proteins which are tightly "wrapped" around the DNA. Through acetylation they loosen themselves, and the DNA can then be transcribed by the transcription machinery. If histone acetylation is lowered, fewer genes are transcribed and accordingly fewer proteins synthesised, such as uncoupling protein UCP-1, which is essential for the activity of brown fat cells.
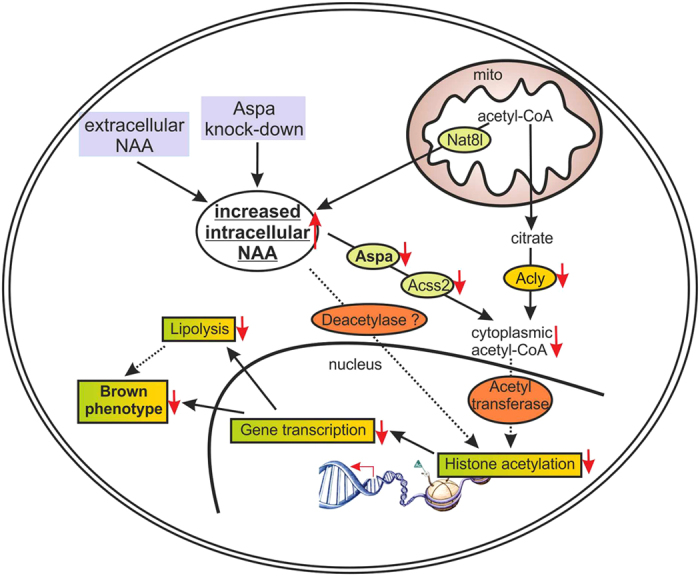
NAA signal path as a possible therapeutic point of attack
The results of the study are especially relevant for research into obesity and diabetes since altered NAA values have been measured in the urine of persons with obesity and type 2 diabetes. "What is particularly interesting here is that NAA occurs in foodstuffs and can enter the fat cells through food intake and could thus influence signal paths," explains Bogner-Strauss. In vivo studies in cooperation with colleagues at the Max Planck Institute for Immunology and Epigenetics in Freiburg could soon shed more light on the matter. "In any case, our current results highlight the signal path as a possible target for developing therapeutic interventions in obesity research," concludes Bogner-Strauss.
Kontakt
Assoc.Prof. Mag.rer.nat. Dr.rer.nat.
Institute of Biochemistry
Humboldtstrasse 46/3
8010 Graz, Austria
Phone: +43 316 380 4970
<link int-link-mail window for sending>juliane.bogner-strauss@tugraz.at
<link https: www.tugraz.at projekte cellism _blank int-link-external external link in new>www.tugraz.at/projekte/cellism/